
The structure of diborane contains:
A.Four $\left( {2C - 2{e^ - }} \right)$ bonds and two $\left( {2C - 2{e^ - }} \right)$ bonds
B.Two $\left( {2C - 2{e^ - }} \right)$ bonds and two $\left( {3C - 2{e^ - }} \right)$ bonds
C.Four $\left( {2C - 2{e^ - }} \right)$ bonds and four $\left( {3C - 2{e^ - }} \right)$ bonds
D.None of these
Answer
589.8k+ views
Hint: A chemical compound, which comprises boron and hydrogen and has a chemical formula of ${B_2}{H_6}$ is diborane. It has no color, and a pyrophoric gas. Other names of diborane are bromoethane, diboron hexahydride and boron hydride. The molecular mass of diborane is $27.67\,g/mol.$
Complete step by step answer:
Based on the molecular orbital theory, the hybridization on each of the two boron atoms is $s{p^3}.$ Of the four orbitals that are hybrid, three orbitals have one electron each whereas the fourth orbital is empty. Two of the four orbitals of each boron atom overlap with two end hydrogen atoms producing two normal B-H sigma bonds. One of the remaining hybrid orbital of one of the boron atoms, 1s orbital of hydrogen atoms and one of hybrid orbitals of the other boron atoms overlap to give a delocalized orbital casing the three nuclei with a pair of electrons. Such a bond is called a three center two electron $\left( {3C - 2e} \right)$ bond.
We can draw the structure of diborane as,
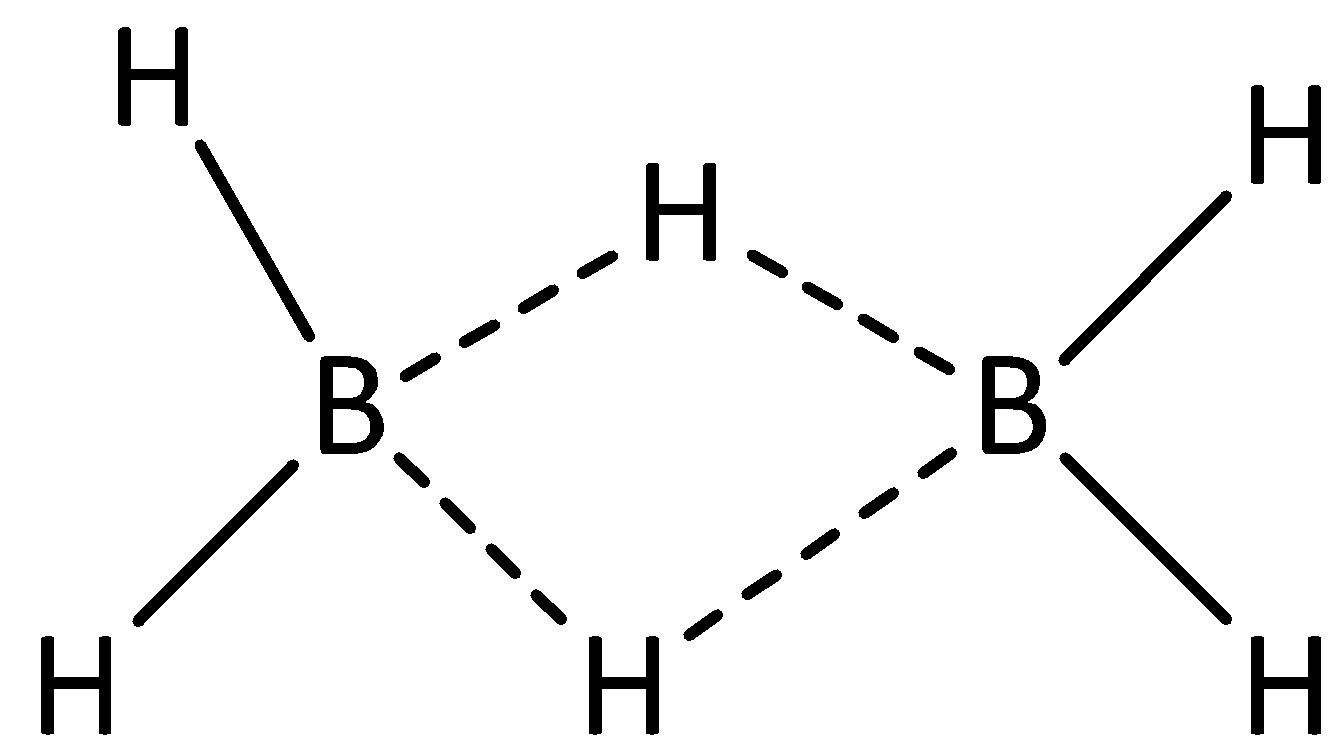
From the structure of diborane, we can see that four atoms of hydrogen are attached to atoms of boron by means of covalent bond. Hence, there are four 2-centered 2-electron bonds.
We can see that two hydrogen atoms are linked via bridges, and it is linked to two atoms of boron. Hence, there are two 3-centered-2-electron bonds.
$\left( {H - B} \right)\xrightarrow{{}}2C - 2e$ bonds
$\left( {B - H - B} \right)\xrightarrow{{}}3C - 2e$ bonds
The 3C-2e bond is also known as banana bond.
$\therefore $Option (D) is correct.
Note:
We know that diborane is flammable gas at room temperature. It has a sweet odor. Diborane on combustion releases a large amount of energy. It can be prepared by the reaction of metal hydride with boron. It reacts with methanol to form trimethyl borate. We can use diborane as propellant in a rocket, used in production of borophosphosilicate, used as a reducing agent. It could act as catalyst and as rubber vulcanizer in polymerization reactions. It is used as a doping agent in manufacturing semiconductor devices.
Complete step by step answer:
Based on the molecular orbital theory, the hybridization on each of the two boron atoms is $s{p^3}.$ Of the four orbitals that are hybrid, three orbitals have one electron each whereas the fourth orbital is empty. Two of the four orbitals of each boron atom overlap with two end hydrogen atoms producing two normal B-H sigma bonds. One of the remaining hybrid orbital of one of the boron atoms, 1s orbital of hydrogen atoms and one of hybrid orbitals of the other boron atoms overlap to give a delocalized orbital casing the three nuclei with a pair of electrons. Such a bond is called a three center two electron $\left( {3C - 2e} \right)$ bond.
We can draw the structure of diborane as,

From the structure of diborane, we can see that four atoms of hydrogen are attached to atoms of boron by means of covalent bond. Hence, there are four 2-centered 2-electron bonds.
We can see that two hydrogen atoms are linked via bridges, and it is linked to two atoms of boron. Hence, there are two 3-centered-2-electron bonds.
$\left( {H - B} \right)\xrightarrow{{}}2C - 2e$ bonds
$\left( {B - H - B} \right)\xrightarrow{{}}3C - 2e$ bonds
The 3C-2e bond is also known as banana bond.
$\therefore $Option (D) is correct.
Note:
We know that diborane is flammable gas at room temperature. It has a sweet odor. Diborane on combustion releases a large amount of energy. It can be prepared by the reaction of metal hydride with boron. It reacts with methanol to form trimethyl borate. We can use diborane as propellant in a rocket, used in production of borophosphosilicate, used as a reducing agent. It could act as catalyst and as rubber vulcanizer in polymerization reactions. It is used as a doping agent in manufacturing semiconductor devices.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




