
The oxidation number and cov-alency of sulphur in sulphur molecule (${{\text{S}}_{8}}$) are:
(A) 0 and 2
(B) +6 and 8
(C) 0 and 8
(D) None of these
Answer
598.5k+ views
Hint: The oxidation state of sulfur is same as that of oxidation state of oxygen atom in its elemental state and covalency can be determined by the drawing the structure of ${{\text{S}}_{8}}$. It is the number of bonds an atom can form within a molecule.
Complete step by step solution:
First of all, we should know about the elemental state of an atom
The elemental state is defined as having all electrons in the ground state configuration. And as per the rule of oxidation number, the oxidation number is zero for an atom in its elemental form.
We can take an example-
Oxygen exists as ${{\text{O}}_{2}}$ molecule in its elemental state and its oxidation number is zero in this state. Otherwise, the oxidation number of oxygen atoms in its ionic form is equal to their ionic charge that is 2.
Apart from this, covalency is the number of covalent bonds that a particular atom can make with other atoms in forming a molecule.
We can take an example-
The simplest atom, hydrogen, has the tendency to form one bond and it can react with another atom to form a molecule. Therefore, hydrogen has covalency of 1. Similarly, oxygen atoms can form two bonds and nitrogen atoms can form three within a molecule.
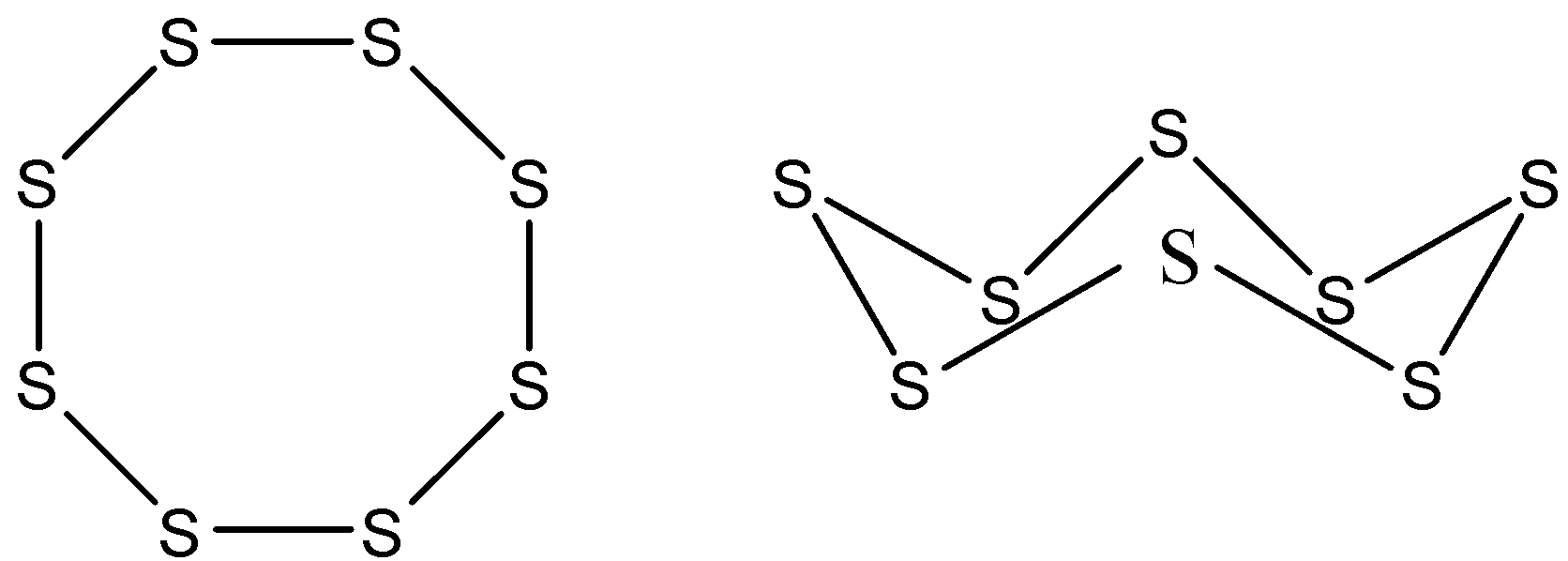
Now, coming back to the sulfur, the structure of the ${{\text{S}}_{8}}$ is given below-
Therefore, the correct option is A. 0 and 2
Note: Do not get confused with the fact that the oxidation number of sulphur is also -2 as sulphur belongs to the oxygen group and oxygen has oxidation number of -2. Also,Sulfur molecule exists in two forms-the stable form at room temperature is called Rhombic sulphur and when it is heated slowly above ${{95}^{\text{o}}}\text{C}$, it is transformed to Monoclinic sulfur.
Complete step by step solution:
First of all, we should know about the elemental state of an atom
The elemental state is defined as having all electrons in the ground state configuration. And as per the rule of oxidation number, the oxidation number is zero for an atom in its elemental form.
We can take an example-
Oxygen exists as ${{\text{O}}_{2}}$ molecule in its elemental state and its oxidation number is zero in this state. Otherwise, the oxidation number of oxygen atoms in its ionic form is equal to their ionic charge that is 2.
Apart from this, covalency is the number of covalent bonds that a particular atom can make with other atoms in forming a molecule.
We can take an example-
The simplest atom, hydrogen, has the tendency to form one bond and it can react with another atom to form a molecule. Therefore, hydrogen has covalency of 1. Similarly, oxygen atoms can form two bonds and nitrogen atoms can form three within a molecule.
Now, coming back to the sulfur, the structure of the ${{\text{S}}_{8}}$ is given below-
Therefore, the correct option is A. 0 and 2

Note: Do not get confused with the fact that the oxidation number of sulphur is also -2 as sulphur belongs to the oxygen group and oxygen has oxidation number of -2. Also,Sulfur molecule exists in two forms-the stable form at room temperature is called Rhombic sulphur and when it is heated slowly above ${{95}^{\text{o}}}\text{C}$, it is transformed to Monoclinic sulfur.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




