
The major product obtained on the interaction of phenol with sodium hydroxide and carbon dioxide is?
A.Benzoic acid
B.Salicylaldehyde
C.Salicylic acid
D.Phthalic acid
Answer
590.1k+ views
Hint: We know that reaction of phenol with sodium hydroxide and carbon dioxide is Kolbe’s Process and the final product of the Kolbe Schmitt reaction is an aromatic hydroxy acid.
Complete step by step answer:
First, we understand Kolbe’s Schmitt reaction.
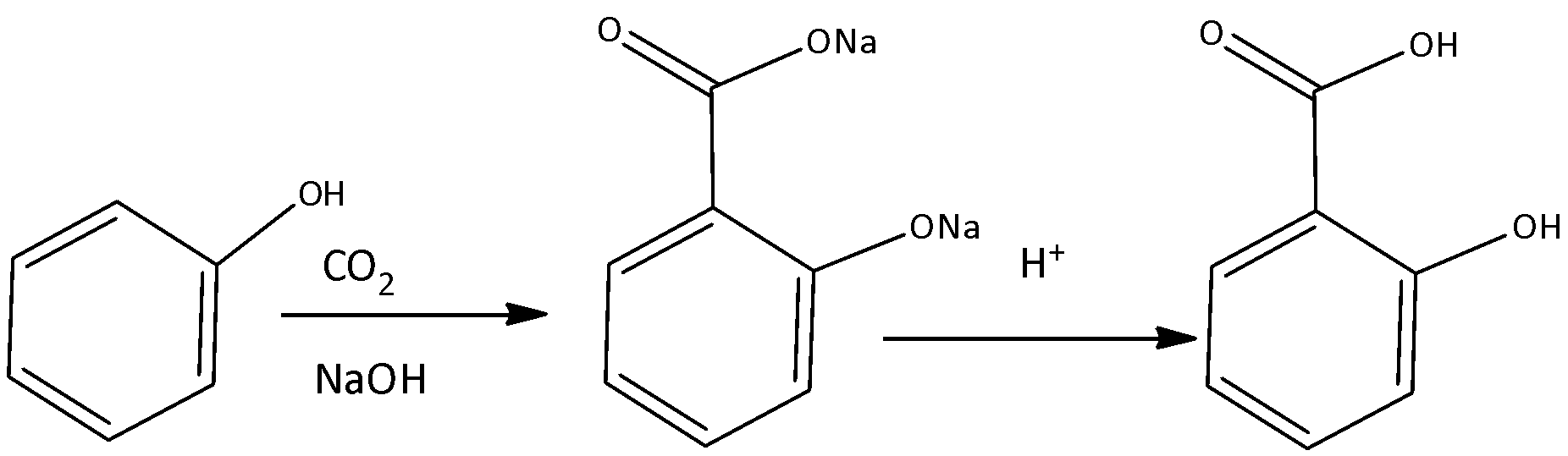
Kolbe’s process is a carboxylation reaction which proceeds by heating the sodium salts of phenoxide with carbon dioxide under pressure and then treated with an acid. The final product of the reaction is an aromatic hydroxy acid.
If Phenol is treated with sodium hydroxide it generates sodium phenoxide ion. The formed phenoxide ion is more reactive than phenol so it undergoes electrophilic aromatic substitution with carbon dioxide which is a weak electrophile and ortho hydroxybenzoic acid is formed as the major product.
$\therefore $Option (C) is the correct answer.
Note:
We must know that in the reaction mechanism of Kolbe’s Schmitt reaction there is the nucleophilic addition of sodium phenoxide with carbon dioxide to form salicylate.
Application of Kolbe’s Schmitt reaction:
-We can use the precursor of parabens (parahydroxybenzoate) as a biocide in cosmetic products is prepared by using the potassium hydroxide in the Kolbe’s process.
-The reaction is also used for the industrial preparation of 3-hydroxy-2-naphthoic acid which is the starting material to azo dyes and pigments.
-We can prepare aspirin by reacting salicylic acid with acetic anhydride. Aspirin is generally used as a painkiller.
Complete step by step answer:
First, we understand Kolbe’s Schmitt reaction.
Kolbe’s process is a carboxylation reaction which proceeds by heating the sodium salts of phenoxide with carbon dioxide under pressure and then treated with an acid. The final product of the reaction is an aromatic hydroxy acid.
If Phenol is treated with sodium hydroxide it generates sodium phenoxide ion. The formed phenoxide ion is more reactive than phenol so it undergoes electrophilic aromatic substitution with carbon dioxide which is a weak electrophile and ortho hydroxybenzoic acid is formed as the major product.

$\therefore $Option (C) is the correct answer.
Note:
We must know that in the reaction mechanism of Kolbe’s Schmitt reaction there is the nucleophilic addition of sodium phenoxide with carbon dioxide to form salicylate.
Application of Kolbe’s Schmitt reaction:
-We can use the precursor of parabens (parahydroxybenzoate) as a biocide in cosmetic products is prepared by using the potassium hydroxide in the Kolbe’s process.
-The reaction is also used for the industrial preparation of 3-hydroxy-2-naphthoic acid which is the starting material to azo dyes and pigments.
-We can prepare aspirin by reacting salicylic acid with acetic anhydride. Aspirin is generally used as a painkiller.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




