
Prepare the following:
(i) Diethyl ketone
(ii) Acetophenone; by decarboxylation method.
Answer
585.3k+ views
Hint: To prepare the diethyl ketone one can think about the methods which use relevant alcohol for the preparation and a suitable reagent must be used for that. The decarboxylation means a process in which removal of the carbon dioxide group takes place. One can use the reagent used for decarboxylation of acetophenone.
Complete step by step answer:
i) First of all we will prepare the Diethyl ketone: The diethyl ketone has five carbon in it having carbonyl group at the center. Hence, we can obtain this by oxidation of the alcohol group of secondary alcohol i.e. $3 - $pentanol. For this reaction to happen one can use any oxidizing agent such as ${K_2}C{r_2}{O_7}$ and the reaction will go as follows.
$C{H_3} - C{H_2} - CH(OH) - C{H_2}C{H_3} + \left[ O \right]\xrightarrow[{{H_2}S{O_4}}]{{{K_2}C{r_2}{O_7}}}{C_2}{H_5} - CO - {C_2}{H_5} + {H_2}O$
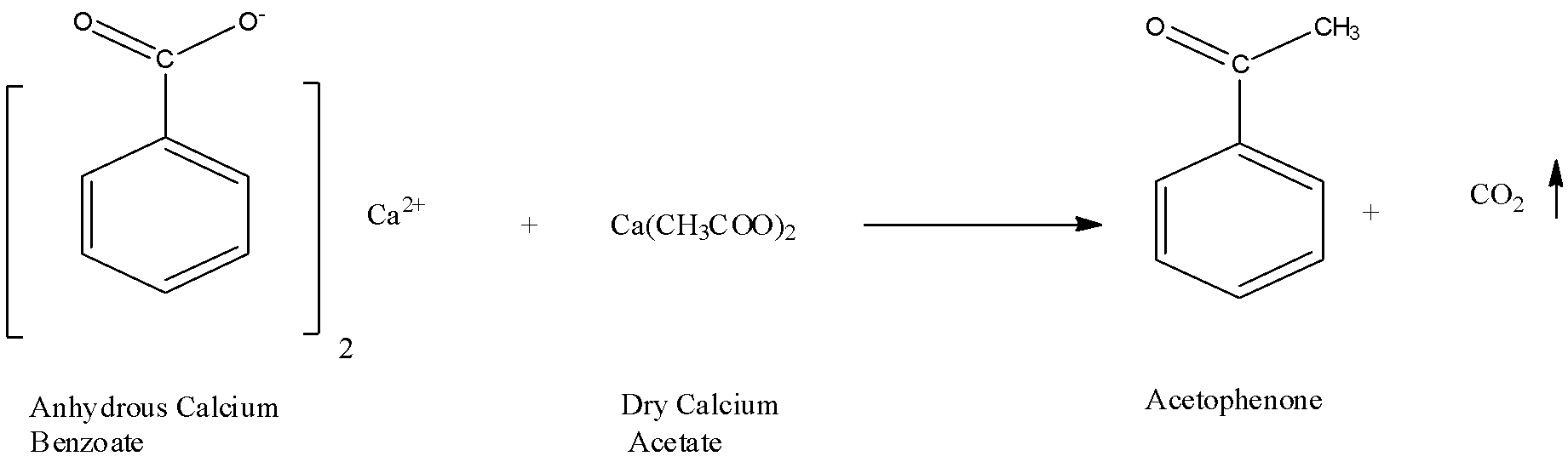
ii) Now let us see the reaction of preparation of acetophenone from the decarboxylation method: The anhydrous calcium benzoate and dry calcium acetate which is acetic acid derivative are blended and this blend is brought into an iron cylinder which is closed toward one side and appended by a long air condenser to a beneficiary. The cylinder is warmed very strongly and the product formed is acetophenone. The decarboxylation means a process in which the removal of the carbon dioxide group happens as a byproduct. The reaction scheme is shown below,
Note:
The oxidizing agent used for the preparation of diethyl ketone is ${K_2}C{r_2}{O_7}$ which is commonly used for oxidation of only primary and secondary alcohols to form relevant aldehydes and ketones respectively. The dry calcium acetate which is a calcium salt of acetic acid and this anhydrous structure is exceptionally hygroscopic which leads to decarboxylation.
Complete step by step answer:
i) First of all we will prepare the Diethyl ketone: The diethyl ketone has five carbon in it having carbonyl group at the center. Hence, we can obtain this by oxidation of the alcohol group of secondary alcohol i.e. $3 - $pentanol. For this reaction to happen one can use any oxidizing agent such as ${K_2}C{r_2}{O_7}$ and the reaction will go as follows.
$C{H_3} - C{H_2} - CH(OH) - C{H_2}C{H_3} + \left[ O \right]\xrightarrow[{{H_2}S{O_4}}]{{{K_2}C{r_2}{O_7}}}{C_2}{H_5} - CO - {C_2}{H_5} + {H_2}O$
ii) Now let us see the reaction of preparation of acetophenone from the decarboxylation method: The anhydrous calcium benzoate and dry calcium acetate which is acetic acid derivative are blended and this blend is brought into an iron cylinder which is closed toward one side and appended by a long air condenser to a beneficiary. The cylinder is warmed very strongly and the product formed is acetophenone. The decarboxylation means a process in which the removal of the carbon dioxide group happens as a byproduct. The reaction scheme is shown below,

Note:
The oxidizing agent used for the preparation of diethyl ketone is ${K_2}C{r_2}{O_7}$ which is commonly used for oxidation of only primary and secondary alcohols to form relevant aldehydes and ketones respectively. The dry calcium acetate which is a calcium salt of acetic acid and this anhydrous structure is exceptionally hygroscopic which leads to decarboxylation.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




