
No indicator can be used for the titration between formic acid and ammonium hydroxide because:
a.) Formic acid is a weak acid and ammonium hydroxide is a weak base.
b.) Formic acid is a weak acid and ammonium hydroxide is a strong base.
c.) Formic acid is a strong acid and ammonium hydroxide is a weak base.
d.) Formic acid is a strong acid and ammonium hydroxide is a strong base.
Answer
601.5k+ views
Hint: In acid-base titrations, a known amount of acid is taken in a conical flask, and the base is taken in the burette. After that, a few drops of the indicator are added. The endpoint is detected by the use of a substance called an acid-base indicator. This indicator shows a sharp change in its color at the end-point.
Complete step-by-step answer:
In acid-base titrations,
Visible end-point: The end-point which is indicated by the change in color of the titration mixture is called the end-point.
Half Equivalence point: The half equivalence point of an acid-base titration is the point at which the concentration of an added base is equal to half of the original concentration of an acid. It is also known as the midpoint of titration.
A weak acid does not donate all of its hydrogen ions ${ H }^{ + }$.
${ HCOOH\rightleftharpoons \quad { HCOO }^{ - } }{ +H }^{ + }$
As we can see that this compound does not release all of its hydrogen ions into the solution, that’s why it is a weak acid.
Ammonium hydroxide is also a weak base as it does not completely dissociate into its ions.
${ NH }_{ 4 }{ OH\rightleftharpoons }{ { NH }_{ 4 }^{ + } }{ +OH }^{ - }$
So, for the titration between a weak acid and a weak base, there is no sharp change in pH.
Hence, no indicator can be used.
The correct option is A.
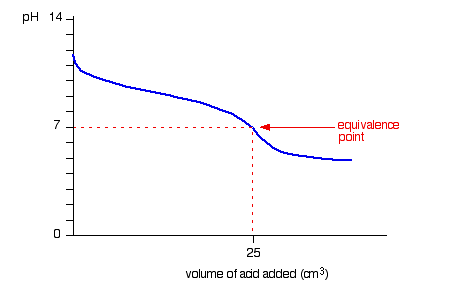
Figure: The graph showing pH vs volume for weak acid -weak base titrations
Note: The possibility of the mistake is that you can choose the option (b). As hydroxide ions are present in ammonium hydroxide but it is not a strong base while is a weak base.
Complete step-by-step answer:
In acid-base titrations,
Visible end-point: The end-point which is indicated by the change in color of the titration mixture is called the end-point.
Half Equivalence point: The half equivalence point of an acid-base titration is the point at which the concentration of an added base is equal to half of the original concentration of an acid. It is also known as the midpoint of titration.
A weak acid does not donate all of its hydrogen ions ${ H }^{ + }$.
${ HCOOH\rightleftharpoons \quad { HCOO }^{ - } }{ +H }^{ + }$
As we can see that this compound does not release all of its hydrogen ions into the solution, that’s why it is a weak acid.
Ammonium hydroxide is also a weak base as it does not completely dissociate into its ions.
${ NH }_{ 4 }{ OH\rightleftharpoons }{ { NH }_{ 4 }^{ + } }{ +OH }^{ - }$
So, for the titration between a weak acid and a weak base, there is no sharp change in pH.
Hence, no indicator can be used.
The correct option is A.

Figure: The graph showing pH vs volume for weak acid -weak base titrations
Note: The possibility of the mistake is that you can choose the option (b). As hydroxide ions are present in ammonium hydroxide but it is not a strong base while is a weak base.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




