
In hcp arrangement , the coordination number is :
A . \[6\]
B . $12$
C . $8$
D . $10$
Answer
598.2k+ views
Hint :We know that metals are made up of atoms. The strength of the metals depends upon the arrangement of their atoms. For the formation of the strongest metallic bonds, metals are packed as closely as possible. Several packing arrangements are possible and hcp is one of them.
Complete step by step solution:
In hcp or hexagonal closed packed arrangement of atoms, the unit cell is made up of three layers of atoms. The Top layers and the bottom layers contain six atoms at the corner of a hexagon and one atom at centre of the hexagon. The middle layer contains three atoms. The middle layer nestled between the atoms of top and bottom layers, hence it is called closed packed structure.
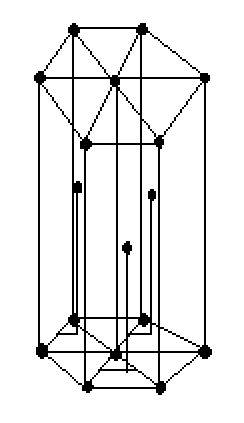
HEXAGONAL CLOSED PACKED STRUCTURE
Cell of hcp lattice consists of seven atoms at the top of the plane and seven atoms at the bottom plane forming a regular hexagon around a central atom. In between these planes there are three atoms which can be seen in the above diagram of hcp arrangement. There is $26\% $ void space in the unit cell of hcp and the rest of the space is efficiently occupied. HCP arrangement has coordination number $12$ and contains six atoms per unit cell.
Hence in the given problem option B is the correct answer that is $12$.
Note : The coordination number can be defined as the number of atoms touching a particular atom in a unit cell. We know that there are six corner atoms in both top and bottom layers and three atoms are within the volume of the lattice .Since each corner atom is common to all unit cells in the hcp ,the contribution of each atom is $1/6$ , the contribution of base centred atom is $1/2$ and three atoms are within the volume . So atoms per unit cell is six and coordination number is twelve.
Complete step by step solution:
In hcp or hexagonal closed packed arrangement of atoms, the unit cell is made up of three layers of atoms. The Top layers and the bottom layers contain six atoms at the corner of a hexagon and one atom at centre of the hexagon. The middle layer contains three atoms. The middle layer nestled between the atoms of top and bottom layers, hence it is called closed packed structure.

HEXAGONAL CLOSED PACKED STRUCTURE
Cell of hcp lattice consists of seven atoms at the top of the plane and seven atoms at the bottom plane forming a regular hexagon around a central atom. In between these planes there are three atoms which can be seen in the above diagram of hcp arrangement. There is $26\% $ void space in the unit cell of hcp and the rest of the space is efficiently occupied. HCP arrangement has coordination number $12$ and contains six atoms per unit cell.
Hence in the given problem option B is the correct answer that is $12$.
Note : The coordination number can be defined as the number of atoms touching a particular atom in a unit cell. We know that there are six corner atoms in both top and bottom layers and three atoms are within the volume of the lattice .Since each corner atom is common to all unit cells in the hcp ,the contribution of each atom is $1/6$ , the contribution of base centred atom is $1/2$ and three atoms are within the volume . So atoms per unit cell is six and coordination number is twelve.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

Differentiate between homogeneous and heterogeneous class 12 chemistry CBSE




