
Explain:
i) Why do transition elements show variable oxidation state?
ii) Give two differences between Lanthanide and Actinide.
Answer
549.3k+ views
Hint: i) Transition elements involve filling up of d- orbitals which have less energy difference with its higher orbitals.
ii.) Lanthanides are the elements of the sixth period starting from lanthanum which involves filling up of 4f orbitals while actinides are the elements of the seventh period starting from actinium which involves filling up 5f orbitals.
Complete step by step answer:
i) The transition metals are also called d- block elements. They show variable oxidation state because transition metals have (n-1)d orbitals empty that are closer to the outermost ns orbital in energy levels. These orbitals are never fully filled. So, they can always accommodate more electrons in (n-1)d orbitals.
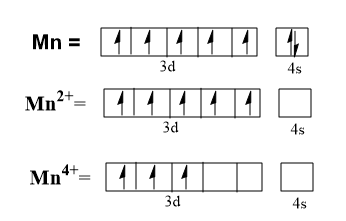
Let’s consider a transition metal, Mn. The atomic number of Mn is 25, hence it has the electronic configuration, $\left[ Ar \right]3{{d}^{5}}4{{s}^{2}}$.
It shows oxidation states from +2 to +7. The generally shown oxidation states are +2, +4 and +7.
To show +2 oxidation state it loses two electrons from the outer 4s orbital and to show +4 oxidation state four electrons are removed from the outer orbitals.
Further, these elements have electrons in two different states of orbitals, i.e. ns and (n-1)d. The energy difference between these ns and (n-1)d orbitals is less. Thus, both can share electrons during bond formation and therefore, both contribute towards bonding.
ii.) Differences between Lanthanides and Actinides.
Note:
i) The stable oxidation state of transition metal is mostly +3. These metals form stable cations. So, they can lose a number of electrons giving them variety in oxidation state.
ii) The lanthanides involves filling of 4f orbitals which is the first f-orbital. Thus, it is also called as the first transition series.
ii.) Lanthanides are the elements of the sixth period starting from lanthanum which involves filling up of 4f orbitals while actinides are the elements of the seventh period starting from actinium which involves filling up 5f orbitals.
Complete step by step answer:
i) The transition metals are also called d- block elements. They show variable oxidation state because transition metals have (n-1)d orbitals empty that are closer to the outermost ns orbital in energy levels. These orbitals are never fully filled. So, they can always accommodate more electrons in (n-1)d orbitals.
Let’s consider a transition metal, Mn. The atomic number of Mn is 25, hence it has the electronic configuration, $\left[ Ar \right]3{{d}^{5}}4{{s}^{2}}$.
It shows oxidation states from +2 to +7. The generally shown oxidation states are +2, +4 and +7.
To show +2 oxidation state it loses two electrons from the outer 4s orbital and to show +4 oxidation state four electrons are removed from the outer orbitals.

Further, these elements have electrons in two different states of orbitals, i.e. ns and (n-1)d. The energy difference between these ns and (n-1)d orbitals is less. Thus, both can share electrons during bond formation and therefore, both contribute towards bonding.
ii.) Differences between Lanthanides and Actinides.
| Lanthanides | Actinides |
| This is the name given to the sixth period in the periodic table. | This is the name given to the seventh period in the periodic table. |
| It involves the filling of 4f and 6s orbital. | It involves filling of 5f and 7s orbital. |
| These show less tendency to form complex compounds. | These have more tendency to form complex compounds. |
| These form compounds which are less basic than actinide compounds. | These form compounds which are more basic in nature. |
| Most of the lanthanides are non-radioactive (except promethium). | All the actinides are radioactive in nature. |
Note:
i) The stable oxidation state of transition metal is mostly +3. These metals form stable cations. So, they can lose a number of electrons giving them variety in oxidation state.
ii) The lanthanides involves filling of 4f orbitals which is the first f-orbital. Thus, it is also called as the first transition series.
Recently Updated Pages
Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

How is the angle of emergence e related to the angle class 12 physics CBSE

Differentiate between lanthanoids and actinoids class 12 chemistry CBSE

Derive Lens Makers formula for a convex lens class 12 physics CBSE

a Draw Labelled diagram of Standard Hydrogen Electrode class 12 chemistry CBSE




