
Esterification of acid chloride with ethanol is usually carried out in the presence of pyridine. The function of pyridine is:
A. To remove HCl formed in the reaction
B. To react with acid chloride to form an acyl pyridinium ion
C. Both A and B
D. As a catalyst
Answer
601.2k+ views
Hint: Think about how the esterification reaction occurs. When an acid chloride molecule reacts with an alcohol molecule to form an ester, think about what role can be played by pyridine in the mechanism.
Complete answer:
We know that pyridine is an important reagent having the molecular formula\[{{\text{C}}_{\text{5}}}{{\text{H}}_{\text{5}}}\text{N}\].
In the first step of this reaction, pyridine will react with acyl chloride to form acyl pyridinium ion. In this step of the reaction, pyridine molecule attacks on the carbonyl carbon and the chlorine in the acid chloride will leave as a good leaving group.
In the next step of the reaction, pyridine will act as a Bronsted-base which will be used to remove the HCl formed in the reaction. So, the function of pyridine in this reaction is to remove the HCl formed in the reaction and also to react with acid chloride to form an acyl pyridinium ion.
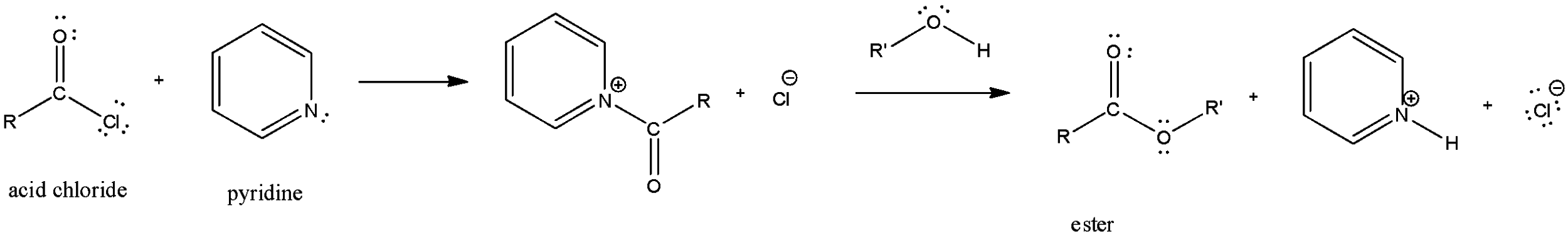
Here, we can clearly see that pyridine first forms the acyl pyridinium and then also removes the $H\text{ and }Cl$.
Hence, the correct answer is ‘C. Both A and B’
Note: You need to have a good knowledge about pyridine. Pyridine is the molecule having the molecular formula \[{{\text{C}}_{\text{5}}}{{\text{H}}_{\text{5}}}\text{N}\] and it is a basic heterocyclic organic compound. One methane group in the benzene is replaced with nitrogen atoms in the pyridine. The lone pair present in the nitrogen atom makes it a good base.
Complete answer:
We know that pyridine is an important reagent having the molecular formula\[{{\text{C}}_{\text{5}}}{{\text{H}}_{\text{5}}}\text{N}\].
In the first step of this reaction, pyridine will react with acyl chloride to form acyl pyridinium ion. In this step of the reaction, pyridine molecule attacks on the carbonyl carbon and the chlorine in the acid chloride will leave as a good leaving group.
In the next step of the reaction, pyridine will act as a Bronsted-base which will be used to remove the HCl formed in the reaction. So, the function of pyridine in this reaction is to remove the HCl formed in the reaction and also to react with acid chloride to form an acyl pyridinium ion.

Here, we can clearly see that pyridine first forms the acyl pyridinium and then also removes the $H\text{ and }Cl$.
Hence, the correct answer is ‘C. Both A and B’
Note: You need to have a good knowledge about pyridine. Pyridine is the molecule having the molecular formula \[{{\text{C}}_{\text{5}}}{{\text{H}}_{\text{5}}}\text{N}\] and it is a basic heterocyclic organic compound. One methane group in the benzene is replaced with nitrogen atoms in the pyridine. The lone pair present in the nitrogen atom makes it a good base.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




