
Dacron (a polymer) is obtained by the condensation polymerization of:
A. Phenol and Formaldehyde
B. Phenol and phthalic acid
C. Terephthalic acid and Formaldehyde
D. Dimethyl terephthalate and ethylene glycol
Answer
591.9k+ views
Hint: Polymerization is a process in which monomers that are small molecules combine and produce a long chainlike molecule called a polymer. There are basically two types of polymerization, that are addition and condensation polymerization.
Complete answer:
- Condensation polymerization is generally where small monomers or we can say molecules react with each other to form polymers that are large structural units. And products like water or methanol is released.
- We can see that Dacron is obtained by the condensation polymerization of Dimethyl terephthalate and ethylene glycol.
- We can see the formation of Dacron from the reaction below:
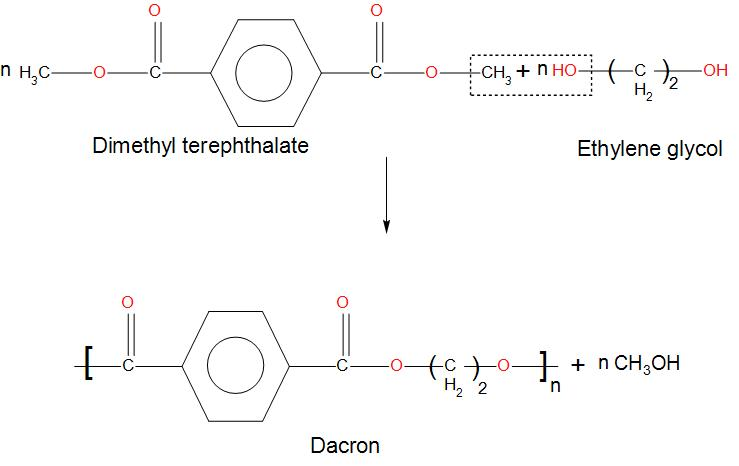
Hence, we can conclude that the correct option is (D) that is Dacron (a polymer) is obtained by the condensation polymerization of Dimethyl terephthalate and ethylene glycol.
Additional information:
- There are various properties found in Dacron like, it is having high resistance to stretching, having high tensile strength.
- It is having wide range of applications, some of them are:
- It is used in manufacturing fibres for clothing.
- Used in making containers for foods and liquids.
- It is also found that Dacron has a wide application in the industrial field. It is used in industries to prepare drive belts and conveyor belts.
Note:
- We should not get confused in the condensation polymerization and addition polymerization. The main difference between both of these is that in condensation polymerization polymers are formed with release of several by products.
- Whereas, in addition polymerization polymers are formed without release of any by product.
Complete answer:
- Condensation polymerization is generally where small monomers or we can say molecules react with each other to form polymers that are large structural units. And products like water or methanol is released.
- We can see that Dacron is obtained by the condensation polymerization of Dimethyl terephthalate and ethylene glycol.
- We can see the formation of Dacron from the reaction below:

Hence, we can conclude that the correct option is (D) that is Dacron (a polymer) is obtained by the condensation polymerization of Dimethyl terephthalate and ethylene glycol.
Additional information:
- There are various properties found in Dacron like, it is having high resistance to stretching, having high tensile strength.
- It is having wide range of applications, some of them are:
- It is used in manufacturing fibres for clothing.
- Used in making containers for foods and liquids.
- It is also found that Dacron has a wide application in the industrial field. It is used in industries to prepare drive belts and conveyor belts.
Note:
- We should not get confused in the condensation polymerization and addition polymerization. The main difference between both of these is that in condensation polymerization polymers are formed with release of several by products.
- Whereas, in addition polymerization polymers are formed without release of any by product.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




