
How many chloride ions are there around sodium ions in sodium chloride crystals?
$
A)\,3 \\
B)\,8 \\
C)\,4 \\
D)\,6 \\
$
Answer
590.7k+ views
Hint: We have to know the crystal structure of sodium chloride crystal to determine the number of chloride ions present in them.NaCl has a cubic unit cell. It is best thought of as a face-centered cubic array of anions with an interpenetrating fcc cation lattice (or vice-versa). The cell looks the same whether you start with anions or cations on the corners.
Complete step by step answer:
The ions lattice position determines the number of unit cells.
We have to know that crystals of sodium chloride are an example of face centred cubic (or) cubic close packed arrangement.
We can draw the crystal structure of sodium chloride as,
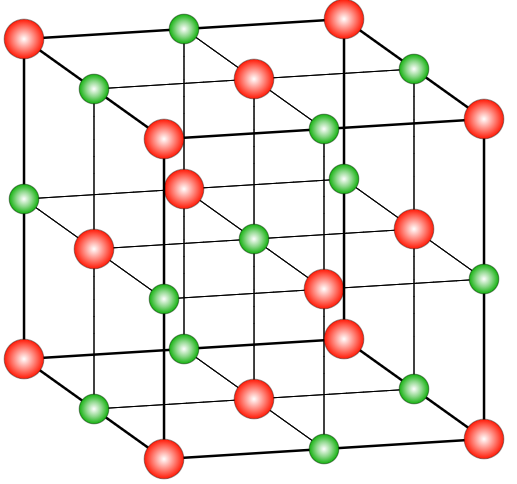
Where red spheres are placed for Chloride ions whereas green spheres are placed for sodium.
We shall understand that in the crystal of face centered cubic structure, there is one atom present at each corner of the cubic unit cell and there is one atom present at the centre of each of the six faces of the cubic unit cell.
Therefore, we have to know that in a crystal structure of sodium chloride, the cubic lattice of sodium ions are interlocked with a similar lattice of chlorine ions.
Each unit cell of sodium chloride contains fourteen chloride ions and thirteen sodium ions.
An ion at a corner is shared by eight unit cells, one at the centre of face is shared by two unit cells and one seen at the edge is shared by four unit cells.
Each ion of chloride is surrounded by six ions of sodium and each ion of sodium is surrounded to six ions of chloride.
Let us also know that the unit cell of sodium chloride has four sodium ions and four chloride ions.
Option (D) is correct.
Note:
The structure of sodium chloride is known as rock-salt structure. Compounds like Lithium hydride $\left( {LiH} \right),$ Sodium iodide $\left( {NaI} \right),$ Potassium chloride $\left( {KCl} \right),$ Rubidium iodide $\left( {RbI} \right),$ Rubidium fluoride $\left( {RbF} \right),$ Lead sulphide $\left( {PbS} \right)$ shows similar structure to sodium chloride.
Complete step by step answer:
The ions lattice position determines the number of unit cells.
We have to know that crystals of sodium chloride are an example of face centred cubic (or) cubic close packed arrangement.
We can draw the crystal structure of sodium chloride as,

Where red spheres are placed for Chloride ions whereas green spheres are placed for sodium.
We shall understand that in the crystal of face centered cubic structure, there is one atom present at each corner of the cubic unit cell and there is one atom present at the centre of each of the six faces of the cubic unit cell.
Therefore, we have to know that in a crystal structure of sodium chloride, the cubic lattice of sodium ions are interlocked with a similar lattice of chlorine ions.
Each unit cell of sodium chloride contains fourteen chloride ions and thirteen sodium ions.
An ion at a corner is shared by eight unit cells, one at the centre of face is shared by two unit cells and one seen at the edge is shared by four unit cells.
Each ion of chloride is surrounded by six ions of sodium and each ion of sodium is surrounded to six ions of chloride.
Let us also know that the unit cell of sodium chloride has four sodium ions and four chloride ions.
Option (D) is correct.
Note:
The structure of sodium chloride is known as rock-salt structure. Compounds like Lithium hydride $\left( {LiH} \right),$ Sodium iodide $\left( {NaI} \right),$ Potassium chloride $\left( {KCl} \right),$ Rubidium iodide $\left( {RbI} \right),$ Rubidium fluoride $\left( {RbF} \right),$ Lead sulphide $\left( {PbS} \right)$ shows similar structure to sodium chloride.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




