
Benzotrichloride reacts with milk of lime to form:
(A). Benzal
(B). Benzoic acid
(C). Benzyl alcohol
(D). Phenol
Answer
601.8k+ views
Hint: In orders to deal with this question first we will understand the compound benzotrichloride and we will see its properties and further we will write its reaction and according to it we will determine the required answer.
Complete step by step solution:
Benzotrichloride is a colorless, unstable, penetrating odor hydrocarbon. Benzotrichloride is commonly used in industry for the processing of dyes as a cosmetic ingredient. Its derivatives are used as UV stabilizers in plastic and to manufacture antiseptics and antimicrobial agents. In the presence of moisture, its hydrolyzed vapors (benzoic acid and hydrochloric acid) are highly irritating to the skin and mucous membranes in humans. The assumption that benzotrichloride is a human carcinogen is reasonable.
Benzotrichloride appears to be a pure yellowish colorless liquid with a strong scent. Denser than water and vapors are heavier than air. May be toxic by inhalation or ingestion. Burns skin, eyes, and mucous membranes. Insoluble in water. Used to make dyes and other chemicals.
Reaction is given as-
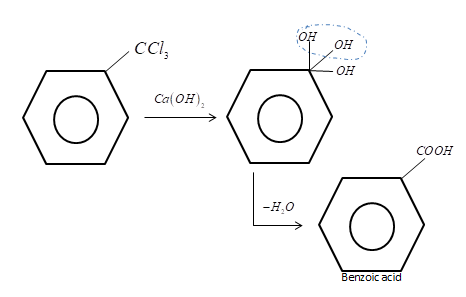
Hence the correct answer is option B.
Note: In the above reaction of milk of lime with benzotrichloride, the milk of lime replaces the chloride ion with the hydroxyl ions and then due to the stability of the hydroxyl group on the same position a water molecule is removed from the intermediate compound. The product at last formed is known as benzoic acid. Lime water can be prepared by mixing milk or lime with water and the excess water can be removed by filtration.
Complete step by step solution:
Benzotrichloride is a colorless, unstable, penetrating odor hydrocarbon. Benzotrichloride is commonly used in industry for the processing of dyes as a cosmetic ingredient. Its derivatives are used as UV stabilizers in plastic and to manufacture antiseptics and antimicrobial agents. In the presence of moisture, its hydrolyzed vapors (benzoic acid and hydrochloric acid) are highly irritating to the skin and mucous membranes in humans. The assumption that benzotrichloride is a human carcinogen is reasonable.
Benzotrichloride appears to be a pure yellowish colorless liquid with a strong scent. Denser than water and vapors are heavier than air. May be toxic by inhalation or ingestion. Burns skin, eyes, and mucous membranes. Insoluble in water. Used to make dyes and other chemicals.
Reaction is given as-

Hence the correct answer is option B.
Note: In the above reaction of milk of lime with benzotrichloride, the milk of lime replaces the chloride ion with the hydroxyl ions and then due to the stability of the hydroxyl group on the same position a water molecule is removed from the intermediate compound. The product at last formed is known as benzoic acid. Lime water can be prepared by mixing milk or lime with water and the excess water can be removed by filtration.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




