
Assertion
Borazole is more reactive than benzene.
Reason
Electrophiles attack at nitrogen and not at boron atoms in inorganic benzene.
(A) The Assertion is true, Reason is true and Reason is the correct explanation for Assertion.
(B) The Assertion is true, Reason is true and Reason is NOT the correct explanation for Assertion.
(C) The Assertion is true, Reason is false.
(D) The Assertion is false, Reason is true.
Answer
592.2k+ views
Hint: Polarity induces reactivity in any compound. Electrophiles are species which are electron-deficient.
Complete answer:
-Borazole is a six-membered heterocyclic compound having alternate boron and nitrogen atoms and alternate double bonds. It is also known as Borazine.
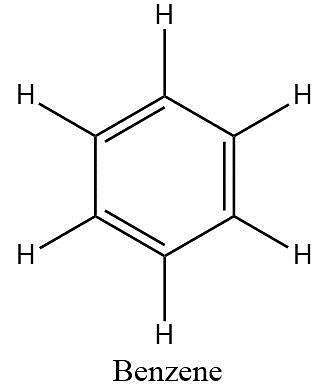
-Borazine is also known as inorganic benzene. It is isostructural to benzene. It contains three double bonds.
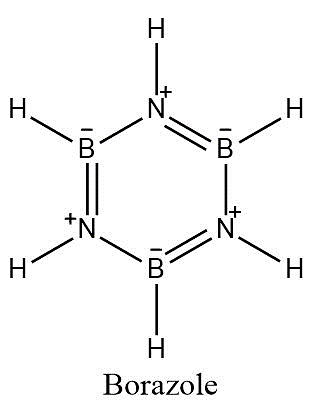
-We are aware that nitrogen is more electron-rich than boron. Nitrogen atoms have one lone pair. Nitrogen donates its lone pair to boron which is electron-deficient which gives rise to double bond between them.
-Now, nitrogen develops a positive charge and boron has a negative charge. So, there is polarity induced in the molecule. Benzene is a non-polar solvent.
-As the polarity increases, reactivity increases and thus, borazole is more polar than benzene. So, the assertion is true.
-When electrophiles attack on borazole, nitrogen atoms donate electrons to the electrophile. Boron being itself electrophilic doesn’t react with electrophiles. So, the reason is also true. But it fails to explain higher stability of benzene in contrast to borazole.
-Therefore, the correct option is option(B) The Assertion is true, Reason is true and Reason is NOT the correct explanation for Assertion.
So, the correct answer is “Option B”.
Note: Benzene and borazine are both resonating structures but due to dissimilar atoms present in borazine, it has less stability. Also, each $N=B$ bond present in a borazine ring is a polar bond which further reduces stability of the entire structure and makes it more reactive.
Complete answer:
-Borazole is a six-membered heterocyclic compound having alternate boron and nitrogen atoms and alternate double bonds. It is also known as Borazine.
-Borazine is also known as inorganic benzene. It is isostructural to benzene. It contains three double bonds.


-We are aware that nitrogen is more electron-rich than boron. Nitrogen atoms have one lone pair. Nitrogen donates its lone pair to boron which is electron-deficient which gives rise to double bond between them.
-Now, nitrogen develops a positive charge and boron has a negative charge. So, there is polarity induced in the molecule. Benzene is a non-polar solvent.
-As the polarity increases, reactivity increases and thus, borazole is more polar than benzene. So, the assertion is true.
-When electrophiles attack on borazole, nitrogen atoms donate electrons to the electrophile. Boron being itself electrophilic doesn’t react with electrophiles. So, the reason is also true. But it fails to explain higher stability of benzene in contrast to borazole.
-Therefore, the correct option is option(B) The Assertion is true, Reason is true and Reason is NOT the correct explanation for Assertion.
So, the correct answer is “Option B”.
Note: Benzene and borazine are both resonating structures but due to dissimilar atoms present in borazine, it has less stability. Also, each $N=B$ bond present in a borazine ring is a polar bond which further reduces stability of the entire structure and makes it more reactive.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




