
Among alcohols and phenols which one is more acidic and why?
Answer
591.9k+ views
Hint: An acid is a molecule or ion that can give a proton (hydrogen ion \[{{\text{H}}^{\text{ + }}}\]) (a Bronsted-Lowry acid), or, alternatively, form a covalent bond with an electron pair (Lewis acid). Acidity is the property, in which a molecule’s ability to lose a proton, is examined. Higher its ability to lose proton higher is the acidity.
Complete answer:
As we know that, Phenol is an organic, aromatic compound with the \[{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{OH}}\] molecular formula. It is a volatile, white crystalline solid. The molecule consists of a group of phenyl \[\left( { - {{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}} \right)\] bonded with a group of hydroxyl \[\left( { - {\text{OH}}} \right)\]. Mildly acidic, it requires careful handling because chemical burns can occur.
In chemistry, alcohol is an organic compound which carries at least one functional hydroxyl group \[\left( { - {\text{OH}}} \right)\] attached to a saturated carbon atom.
Structure of phenol:
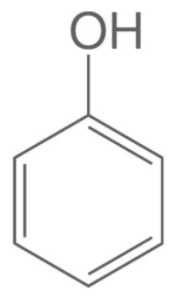
Structure of alcohol:
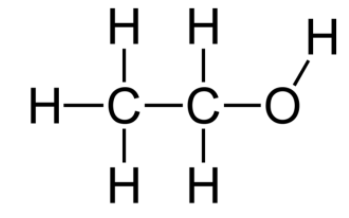
While phenols are stronger acids than alcohols, they are still very weak acids. A standard alcohol carries a \[16 - 17\] \[{\text{p}}{{\text{K}}_{\text{a}}}\]. Phenols react to the phenoxide ions with aqueous sodium hydroxide. This means a higher acidity of phenols relative to alcohols. Because of the resonance in the benzene ring, the phenoxide ion is stabilized by the delocalization of negative charge.
In phenol:
\[{\text{PhOH}} \rightleftharpoons {\text{Ph}}{{\text{O}}^ - } + {{\text{H}}^ + }\]
Phenol is about 1 million times more acidic relative to aliphatic alcohols but it is still considered a weak acid. It responds fully to loss of \[{{\text{H}}^{\text{ + }}}\] with aqueous \[{\text{NaOH}}\], giving the salt sodium phenoxide, whereas most alcohols react only partially.
In the case of any alcohol (e.g. \[{\text{C}}{{\text{H}}_{\text{3}}}{\text{OH}}\]), the alkyl group attached to the oxygen atom is by course releasing electrons due to inductive action \[{\text{ + I}}\] effect. This alkyl group character decreases the electronegativity of oxygen and thus decreases its ability to give \[{{\text{H}}^{\text{ + }}}\].
Thus Phenols are more acidic than alcohols due to resonance.
Note: Phenols are much more acidic than alcohols due to negative charge. Acidic compounds are more negatively charged then phenol and only due to this nature they form more anions with the compounds and carry negative charge and those having negative charge they are more acidic in nature.
Complete answer:
As we know that, Phenol is an organic, aromatic compound with the \[{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{OH}}\] molecular formula. It is a volatile, white crystalline solid. The molecule consists of a group of phenyl \[\left( { - {{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}} \right)\] bonded with a group of hydroxyl \[\left( { - {\text{OH}}} \right)\]. Mildly acidic, it requires careful handling because chemical burns can occur.
In chemistry, alcohol is an organic compound which carries at least one functional hydroxyl group \[\left( { - {\text{OH}}} \right)\] attached to a saturated carbon atom.
Structure of phenol:

Structure of alcohol:

While phenols are stronger acids than alcohols, they are still very weak acids. A standard alcohol carries a \[16 - 17\] \[{\text{p}}{{\text{K}}_{\text{a}}}\]. Phenols react to the phenoxide ions with aqueous sodium hydroxide. This means a higher acidity of phenols relative to alcohols. Because of the resonance in the benzene ring, the phenoxide ion is stabilized by the delocalization of negative charge.
In phenol:
\[{\text{PhOH}} \rightleftharpoons {\text{Ph}}{{\text{O}}^ - } + {{\text{H}}^ + }\]
Phenol is about 1 million times more acidic relative to aliphatic alcohols but it is still considered a weak acid. It responds fully to loss of \[{{\text{H}}^{\text{ + }}}\] with aqueous \[{\text{NaOH}}\], giving the salt sodium phenoxide, whereas most alcohols react only partially.
In the case of any alcohol (e.g. \[{\text{C}}{{\text{H}}_{\text{3}}}{\text{OH}}\]), the alkyl group attached to the oxygen atom is by course releasing electrons due to inductive action \[{\text{ + I}}\] effect. This alkyl group character decreases the electronegativity of oxygen and thus decreases its ability to give \[{{\text{H}}^{\text{ + }}}\].
Thus Phenols are more acidic than alcohols due to resonance.
Note: Phenols are much more acidic than alcohols due to negative charge. Acidic compounds are more negatively charged then phenol and only due to this nature they form more anions with the compounds and carry negative charge and those having negative charge they are more acidic in nature.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




