Revision Notes on Chemistry in Everyday Life for NEET 2026- Free PDF Download
FAQs on Revision Notes on Chemistry in Everyday Life for NEET 2026
1. What are the uses of Chemistry in Everyday Life?
Chemistry is an important aspect of our daily lives. This discipline of study is plainly visible in several aspects of human existence, including as the food we eat, the air we breathe, and in the different cleaning agents we use.
2. What are different types of Food Additives?
There are different types including-
- Artificial Sweetening Agents: Chemical substances that give food a sweetening effect and enhance its flavour.
- Food Preservatives: Chemical compounds that are added to food to avoid spoiling due to microbial development.
- Nutritional Supplements: These are those substances added to the food that can improve the overall nutritional value.
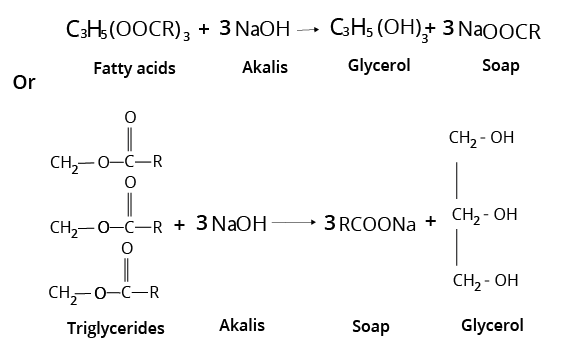
3. What are different types of Soaps?
Toilet soaps are made with higher quality fats and oils, and extra care is taken to remove excess alkali.
- To make transparent soaps, dissolve the soap in ethanol and then evaporate the excess solvent.
- Medicated soaps contain medicinally valuable ingredients. Deodorants are sometimes added to soaps.
- Glycerol is added to shaving soaps to keep them from drying out too quickly. Rosin gum is used in the manufacturing process. It produces sodium rosinate, which lathers nicely.
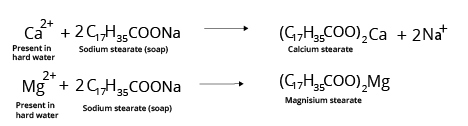
4. What are the advantages of using Soaps?
Soap acts as the cleaning agent that is totally biodegradable, which means that microorganisms in sewage water can completely oxidise soap. As a result, soaps do not contribute to pollution.