




Introduction to Chemistry in Everyday Life
Chemistry's basics have been put to good use for humanity. When you think of cleanliness, soaps, detergents, home bleaches, dental pastes, and other items spring to mind. Look at the lovely garments, and chemicals from the synthetic fibres used to make them, as well as chemicals used to colour them, will quickly spring to mind. Food ingredients – your mind will recall a number of compounds that you learned about in the previous Unit. Sickness and illnesses, of course, conjure up images of remedies — once again, chemicals. Chemicals include explosives, fuels, rocket propellants, construction and electrical materials, and so on. All these chemicals show application of chemistry in everyday life.
Important Topics for Chemistry in Everyday Life
Introduction to Chemistry in Everyday Life
Therapeutic Action of Different Classes of Drugs
Cleansing Agents
Drugs and their Classification
Chemicals in Food
Drug-Target Interaction
Drugs and their Classification
(a) Based on Pharmacological Effect
This categorisation is based on the medications pharmacological effects. It is beneficial to doctors since it provides them with a comprehensive list of medications accessible for the treatment of a certain disease. Antiseptics, for example, destroy or slow the growth of bacteria, whereas analgesics relieve pain. Drugs are the best examples to show the importance of chemistry in everyday life.
(b) Based on Drug Action
It is based on a drug's effect on a certain biochemical process.
For example, antihistamines suppress the function of histamine, a substance that induces inflammation in the body. Histamines' activity can be inhibited in a variety of ways.
(c) Based on Chemical Structure
It is based on the drug's chemical structure. Drugs categorised in this fashion have comparable structural characteristics and pharmacological action.
(d) Based on Molecular Targets
Biomolecules such as carbohydrates, lipids, proteins, and nucleic acids are commonly interacting with drugs. These are referred to as drug targets or target molecules. Drugs with similar structural characteristics may have similar mechanisms of action on their targets. For medicinal chemists, the categorisation based on molecular targets is the most useful.
Drug-Target Interaction
Enzymes as Drug Targets
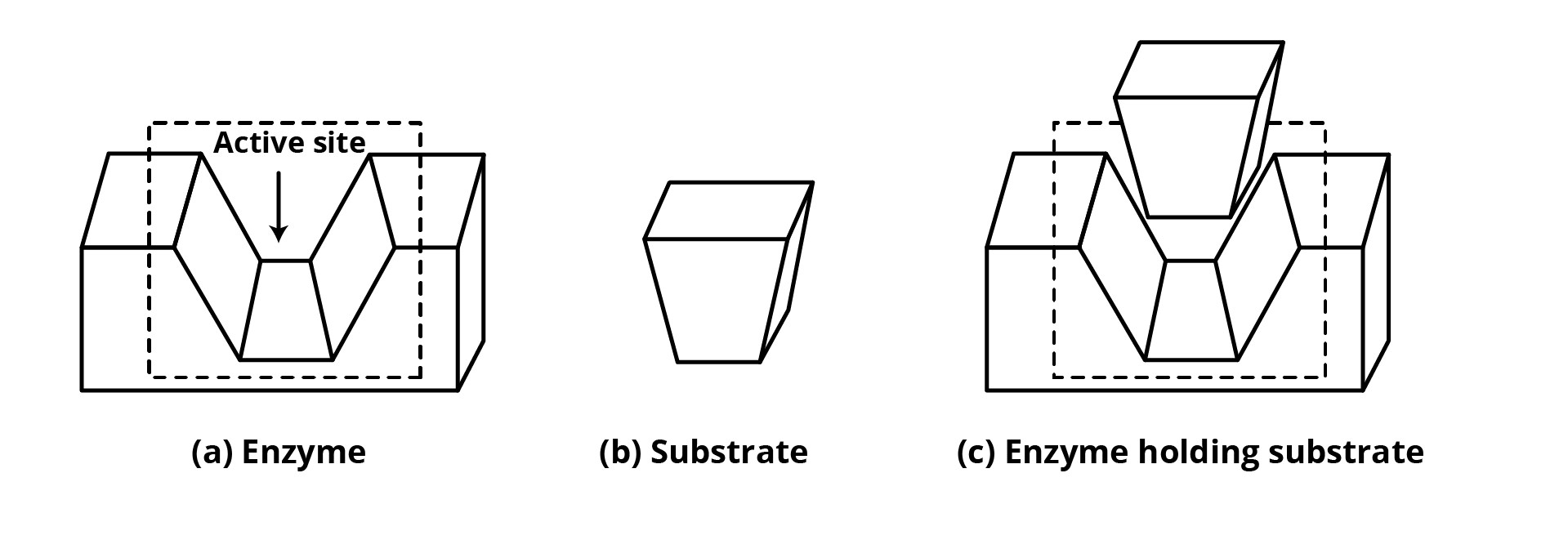
(a) Catalytic Action of Enzymes
Enzyme's first job is to retain the substrate for a chemical reaction. Enzyme active sites keep the substrate molecule in a good place so that the reagent may attack it efficiently. Drugs and substrates compete for the active site in everyday life.
An enzyme's second purpose is to supply functional groups that attack the substrate and carry out chemical reactions.
(b) Drug-Enzyme Interaction:
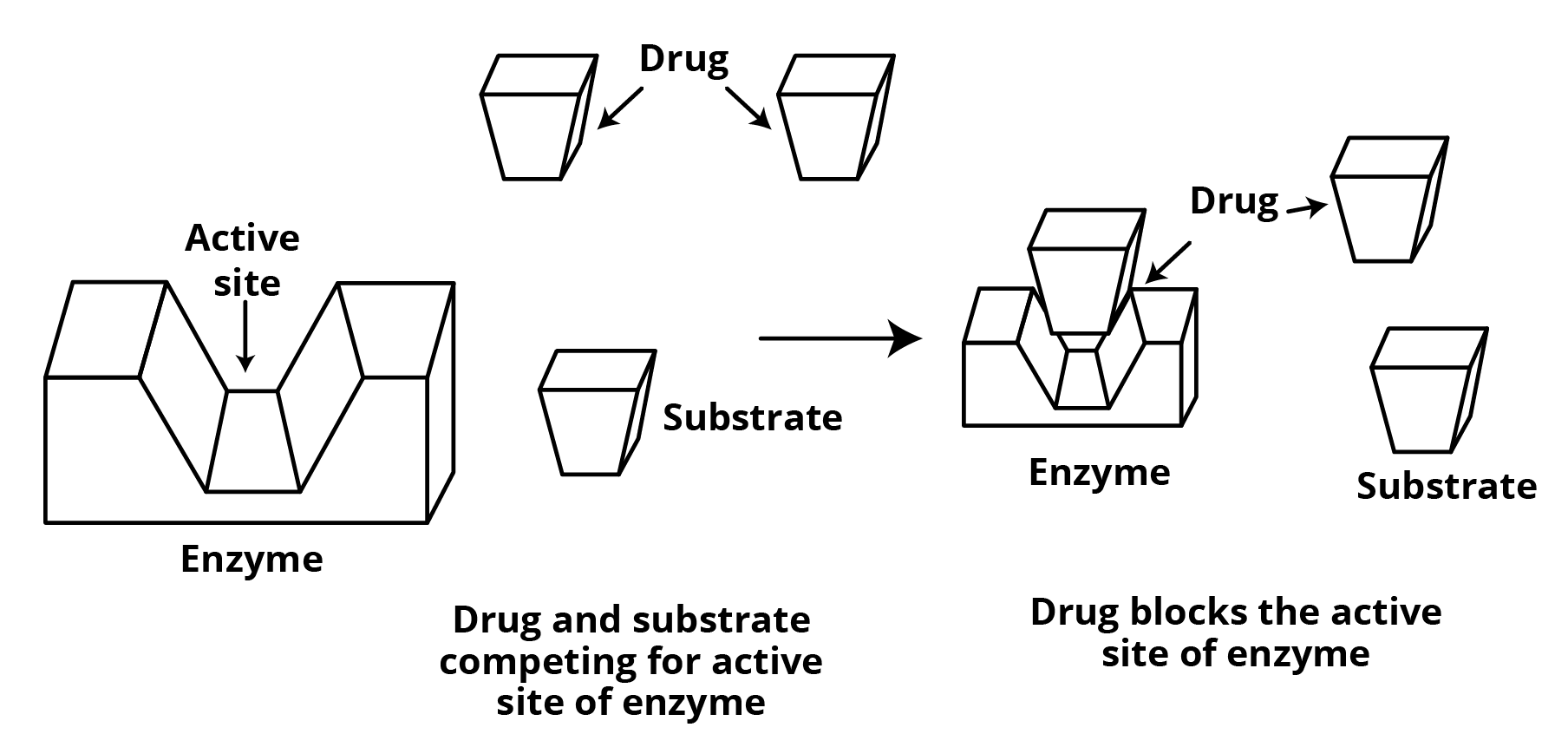
Drugs can inhibit any of the enzyme activities listed above. Blocking the enzyme's binding site may prevent it from binding to substrate, or obstruct the catalytic activity of the enzyme. Enzyme inhibitors are a type of drug that accomplishes this. Drugs work in two ways to prevent substrates from attaching to the active site of enzymes.
(I) Drugs compete with the natural substrate for adhesion. The active sites of enzymes are the locations where enzymes carry out their functions. Inhibitors are known as competitive medications.
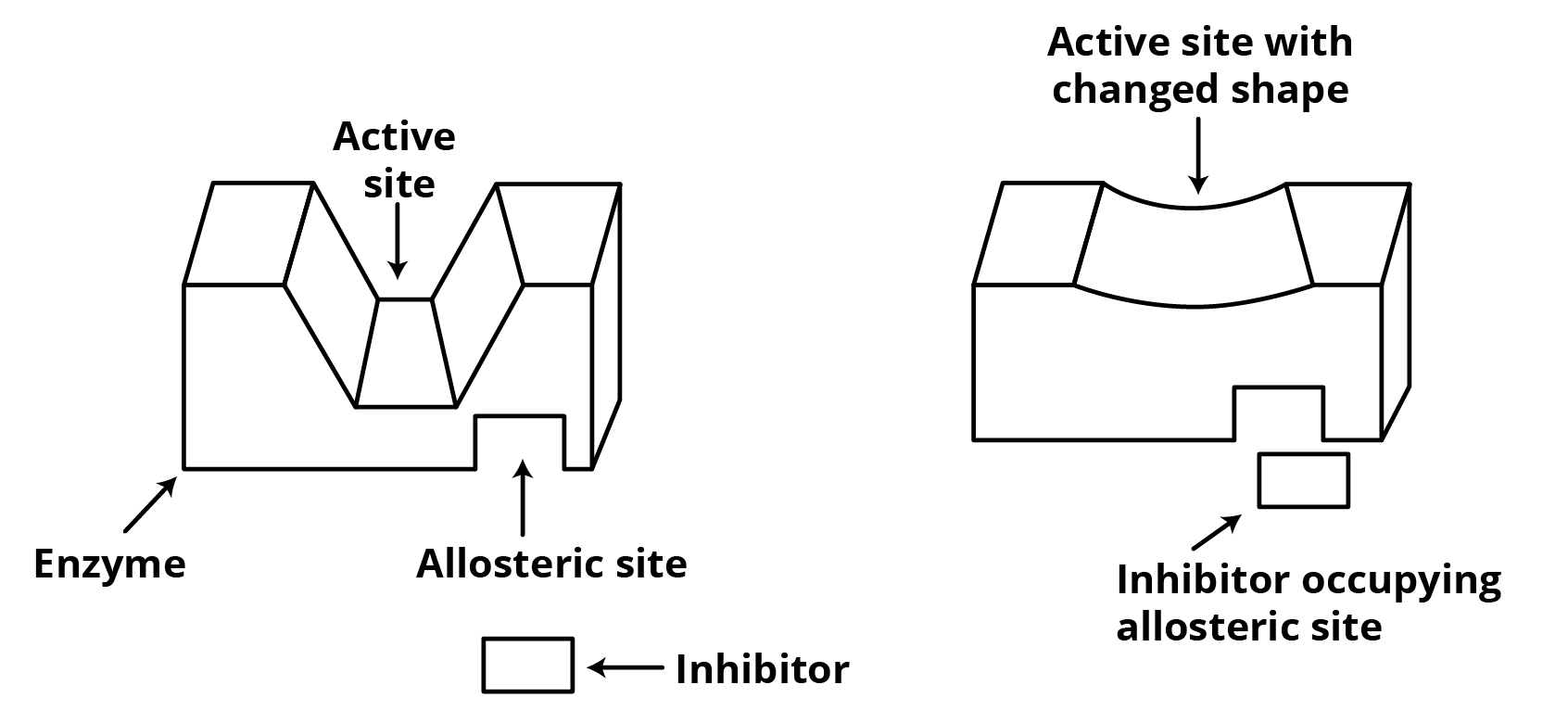
(II) Some medications do not bind to the enzyme's active site. These bind to a specific location on an enzyme. An allosteric site is what this is called. This inhibiting binding occurs at the active sites formed by changes in allosteric location.
Receptors as Drug Targets
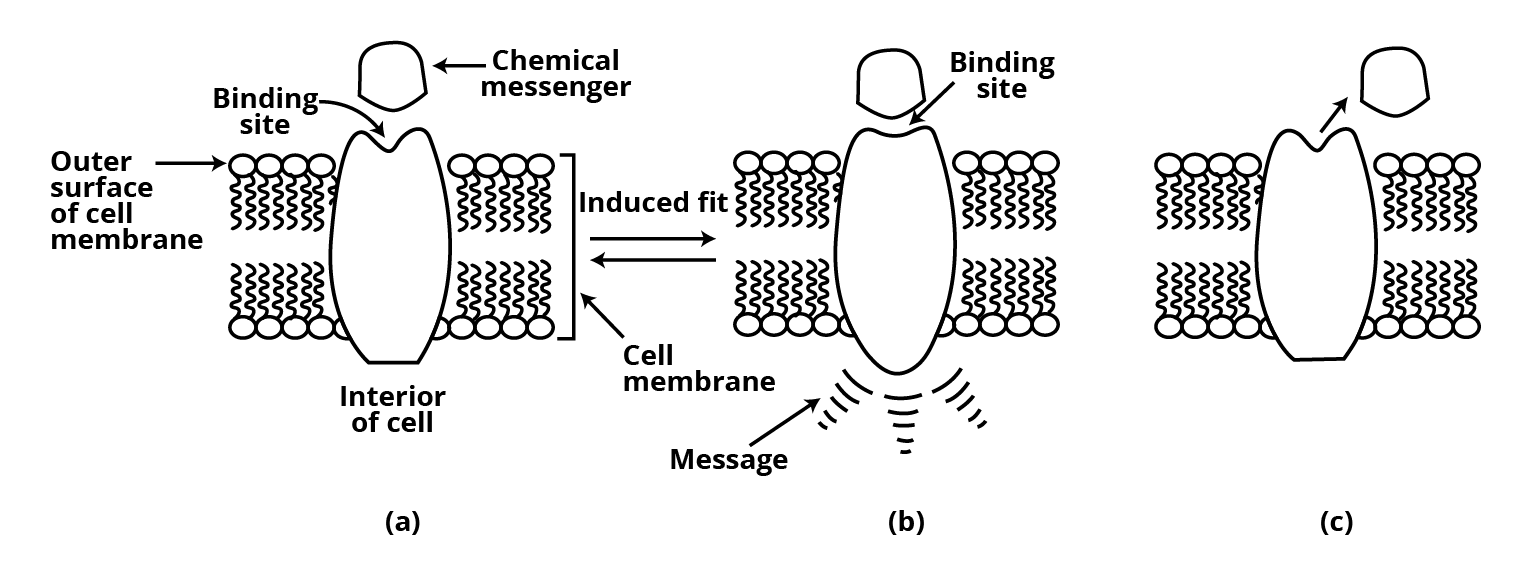
Receptors are proteins that play an important role in the body's communication system. The vast majority of them are found within cell membranes. Receptor proteins are anchored in the cell membrane in such a way that their active site-containing little portion protrudes from the membrane's surface and opens into the cell membrane's outer area.
The active site of the receptor is located on the outside of the cell and is embedded in the cell membrane. The human body contains a huge number of various receptors.
Because of their binding specificity for one chemical messenger over the other, these receptors demonstrate a preference for one chemical messenger over the other.
The shape, structure, and amino acid makeup of various sites vary. The message between two neurons and the message between neurons in the body. Chemicals are used to communicate with muscles. These substances, which are the chemical messengers, are received at receptor binding sites and are referred to as chemical messengers. The shape of the receptor site alters to accommodate a message.
The message gets transferred into the cell as a result of this. As a result, chemical
without entering the cell, the messenger sends a message to it. Drugs that attach to the receptor site and prevent it from performing its normal function are referred to as antagonists. These are useful when communication is being blocked.
Therapeutic Action of Different Classes of Drugs
Antacids
Acid indigestion, acidity, heart burns, and stomach ulcers can all be relieved with antacids, which are chemical chemicals that neutralise excess acid in gastric secretions. Eno, gelusil, digene, and other similar compounds are examples.
Antihistamines
Antihistamines are chemical compounds that reduce the impact of histamine generated in the body, hence preventing allergic responses. Brompheniramine (Dimetapp) and terfenadine are two examples (Seldane.)
Antihistamines prevent allergies by blocking the effect of histamines. Antihistamines are used to treat the symptoms of allergies such as hay fever and food allergies.
Neurologically Active Drugs
(a) Tranquilizers
Analgesics and tranquilizers are both neurologically active medicines. They are chemical substances that are effective in treating stress and moderate to severe mental illnesses. They provide a person with a sense of well-being, relieving him of stress, tension, anxiety, and irritation.
Some tranquillizers, such as chlordiazepoxide and meprobamate, are gentle in nature and can be used to relieve stress. Equanil is a drug that is used to treat depression and hypertension. Valium and serotonin are two chemicals that are utilised as tranquillizers.
(b) Analgesics
Analgesics lessen or eliminate pain without impairing awareness, producing mental disorientation, incoordination, paralysis, or causing other nervous system disruptions. The following are the categories:
(I) Non-addictive (non-narcotic) analgesics
(II) Narcotic substances
(I) Non-addictive (non-narcotic) analgesics: Non-narcotic analgesics, such as aspirin and paracetamol, are used to relieve pain.
The most well-known example is aspirin. Aspirin prevents the production of molecules called prostaglandins, which cause tissue inflammation and discomfort. These medications are beneficial in treating skeletal pain, such as arthritic pain. Other effects of these medications include lowering fever (antipyretic) and inhibiting platelet coagulation. Aspirin is used to prevent heart attacks because of its anti-clotting properties.
(II) Narcotic substances: Morphine and several of its homologues, when given in therapeutic quantities, ease pain and generate euphoria. These cause stupor, coma, and convulsions at toxic doses and, eventually, death. Morphine narcotics are also known as morphine narcotics. Because they are derived from the opium poppy, they are referred to as opiates. These analgesics are primarily used to alleviate postoperative pain, discomfort, including heart pain, cancer-related pain, and pain after childbirth. Morphine and several of its homologues are narcotic analgesics.
(c) Antimicrobials
Infection and illness are caused by several types of organisms such as bacteria, fungus, and viruses. An antimicrobial agent is a medicine that prevents microorganisms from becoming harmful. Antibiotics, antiseptics, and disinfectants are among the examples.
(I) Antibiotics
Antibiotics are chemicals obtained from one microbe that is used to destroy another microorganism. Antibiotics can help with bacterial, fungal, and parasitic infections, but they can't help with viral infections. Ampicillin and amoxicillin are two examples.
Antibiotics may be classified into two categories:
(I) Antibiotics that destroy germs are known as bactericidal antibiotics. Penicillin, aminoglycosides, and ofloxacin are examples of antibiotics.
(II) Antibiotics that suppress microorganisms are known as bacteriostatic antibiotics. Erythromycin, Tetracycline, and Chloramphenicol are other examples.
Antibiotics are categorised into three kinds based on their range of action. The following are some of them:
(I) Antibiotics with a broad spectrum of action are commonly used to kill or inhibit Gram-positive and Gram-negative microorganisms. Chloramphenicol is a good example.
(II) Antibiotics with a narrow spectrum of action: These antibiotics are effective against only a few types of bacteria. Penicillin G is an example. These antibiotics are only effective against a specific organism or illness. Dysidazirine is an example.
(II) Antiseptic and Disinfectants
Antiseptics are used to treat live tissues such as wounds, cuts, ulcers, and sick skin. Furacine, soframycin, and other similar compounds are examples. Antibiotics are consumed, but these aren't. Dettol is a combination of chloroxylenol and terpineol that is commonly used as an antiseptic.
Bithionol (also known as bithional) is a chemical that is added to soaps to give them antibacterial qualities.
Iodine is a highly effective antiseptic. The tincture of iodine is a solution of 2-3% iodine in an alcohol-water combination. It is used to treat wounds. Iodoform is also utilised as a wound antiseptic. Boric acid is a mild antiseptic for the eyes in a dilute aqueous solution.
Inanimate items such as flooring, drainage systems, instruments, and so on are disinfected with disinfectants. By altering the concentration, the same chemicals can serve as both an antiseptic and a disinfectant. For example, a 0.2 percent phenol solution is antiseptic, whereas a one percent phenol solution is a disinfectant.
Disinfectants include chlorine, which has a concentration of 0.2 to 0.4 ppm in aqueous solution, and sulphur dioxide, which has a concentration of very low concentrations.
(d) Antifertility Drugs
People have lived longer and healthier lives as a result of the antibiotic revolution. The average lifespan has nearly doubled. Increased population has resulted in a slew of socioeconomic difficulties, including food scarcity, environmental concerns, and work opportunities. Controlling the population is necessary to address these issues.
As a result, the notion of family planning was born. In this case, antifertility medicines can help. The majority of birth control tablets are made up of a combination of synthetic oestrogen and progesterone derivatives. Hormones are both of these substances. Ovulation is known to be suppressed by progesterone. Synthetic progesterone derivatives have a higher potency than progesterone itself.
Norethindrone is a synthetic progesterone derivative that is commonly used as an antifertility medication. Ethynylestradiol is an oestrogen derivative that is combined with a progesterone derivative (novestrol).
Chemicals in Food
Application of chemistry in everyday life can be demonstrated in the form of food additives. Chemicals are used in food (i) to preserve it, (ii) to make it more appealing, and (iii) to increase its nutritional value. The following are the main categories of food additives:
(i) Food colouring
(ii) Flavours and sweeteners
(iii) Fat emulsifiers and stabilisers
(iv) Flour improvers - antistaling agents and bleaches
(v) Antioxidants
(vi) Preservatives
(vii) Nutritional supplements such as minerals, vitamins, and amino acids
All of the aforementioned additions, with the exception of compounds in category (vii), have no nutritional benefit. These are used to extend the shelf life of stored food or for aesthetic reasons. Only sweeteners and food preservatives will be discussed in this section.
(i) Artificial Sweetening Agents
Because natural sweeteners, such as sucrose, increase calorie consumption, many people choose to utilise artificial sweeteners. Saccharin, also known as ortho-sulfabenzamide, was the first widely used artificial sweetening ingredient. Since its discovery in 1879, it has been employed as a sweetening agent. It's 550 times sweeter than cane sugar. It remains unmodified when it leaves the body and is eliminated in urine. When ingested, it appears to be completely inactive and harmless. Its usage is extremely beneficial to diabetics and individuals who need to keep their calorie consumption under control.
The most popular and commonly used artificial sweetener is aspartame. It's around 100 times sweeter than cane sugar. It's a dipeptide made up of aspartic acid and phenylalanine that's methyl esterified. Because aspartame is unstable at cooking temperatures, it is only used in cold meals and soft drinks.
Alitame is a high-potency sweetener, while more stable than aspartame, makes controlling the sweetness of food challenging.
Sucralose is a sucrose trichloro derivative. It has a sugary look and flavour. At cooking temperature, it remains stable. It does not contain any calories.
(ii) Food Preservatives
Food preservatives keep food from spoiling due to microbial development.
Table salt, sugar, vegetable oils, and sodium benzoate, C6H5COONa, are the most often used preservatives. Sodium benzoate is used in small amounts and is broken down by the body. Preservatives such as sorbic acid salts and propanoic acid salts are also utilised.
(iii) Antioxidants in Food
These are required and useful food additives. These aid in food preservation by preventing oxygen from reacting with the food. They're more reactive to oxygen than the food they're protecting. Butylated hydroxytoluene (BHT) and butylated hydroxyanisole (BHA) are the two most well-known antioxidants (BHA). When BHA is added to butter, it extends its shelf life from months to years.
To increase the action, BHT and BHA are sometimes combined with citric acid. Wine and beer, sugar syrups, and chopped, peeled, or dried fruits and vegetables all benefit from sulphur dioxide and sulphite as antioxidants.
Cleansing Agents
(i) Soaps
Soaps have been used as detergents for a long time. Soaps made from sodium or potassium salts of long-chain fatty acids, such as stearic, oleic, and palmitic acids, are used for cleaning. Soaps with sodium salts are made by heating fat (the glyceryl ester of fatty acid) with an aqueous sodium hydroxide solution. Saponification is the name for the above explained reaction.
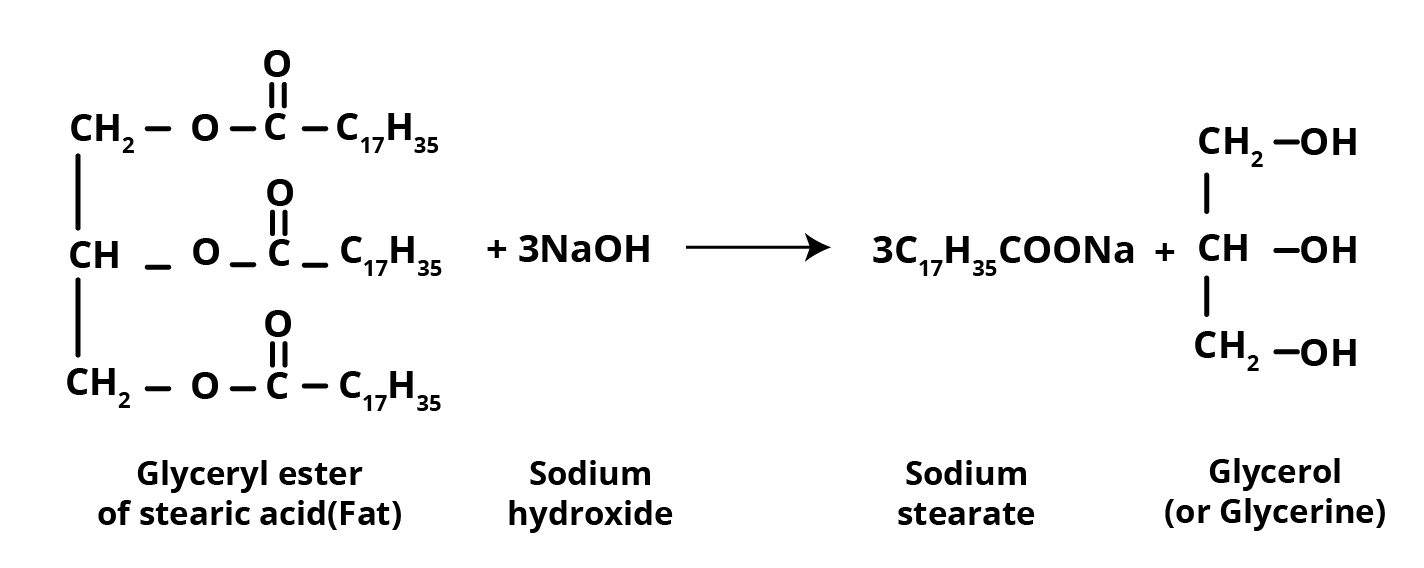
Esters of fatty acids are hydrolysed in this process, and the soap produced is colloidal. Sodium chloride is basically used to precipitate it from the solution. Glycerol may be recovered by fractional distillation from the solution left after removing the soap.
Soaps containing sodium and potassium are the only ones that are soluble in water and may be used for cleaning. Potassium soaps are often gentler on the skin than sodium soaps.
Types of Soap
Almost all soaps are created by heating fats or oils with the right ingredients.
(i) Toilet soaps are made with higher-quality fats and oils and extra alkali is removed with care. Colours and scents are important.
(ii) Floating soaps are formed by beating small air bubbles together before they become brittle. By dissolving the soap in water, transparent soaps can be created.
Calcium and magnesium ions are found in hard water. When sodium or magnesium ions combine, they generate insoluble calcium and magnesium soaps, respectively. In hard water, potassium soaps dissolve. In water, these insoluble soaps separate as scum and are worthless.
These, in reality, constitute a hindrance to effective cleaning because the precipitate forms a sticky coating on the cloth's fibres. Because of this sticky residue, hair cleaned with harsh water appears drab. On a soap-washed fabric, the dye does not soak uniformly. Dye does not absorb evenly on cloth washed with the soap using hard water, because of this gummy mass.
(ii) Synthetic Detergents
Synthetic detergents are cleaning chemicals that have all of the qualities of soap but don't include any soap. These may be used in both soft and hard water since they produce foam in both. Even in ice-cold water, certain detergents produce foam. Synthetic detergents are primarily divided into three groups:
(a) Anionic Detergent
Sulphonated long-chain alcohols or hydrocarbons are sodium salts of anionic detergents. To make anionic detergents, alkyl hydrogen sulphates are generated by processing long-chain alcohols with strong sulphuric acid and then neutralised with alkali.
The cleaning activity of anionic detergents is mediated by the anionic portion of the molecule. An important type of anionic detergent is sodium salts of alkylbenzene sulfonates.
(b) Cationic Detergents
Quaternary ammonium salts of amines with acetates, chlorides, or bromides as anions are referred to as cationic detergents. A lengthy hydrocarbon chain and a positive charge on the nitrogen atom characterise cationic components. Cationic detergents are so named because of this.
Hair conditioners include cetyltrimethylammonium bromide, a common cationic detergent. Cationic detergents are employed in hospitals because they have germicidal qualities.
(c) Non-ionic Detergents
Non-ionic detergents are made up of molecules that do not include any ions. When stearic acid interacts with polyethylene glycol, a detergent is created. Non-ionic liquid dishwashing detergents are available.
(d) Biodegradable and Non-biodegradable Detergents
Detergents having straight hydrocarbon chains that are easily destroyed by microorganisms are known as biodegradable detergents. For example, Sodium lauryl sulphate.
Non-biodegradable detergents are those that have branched hydrocarbon chains that are difficult for microorganisms to break down.
Solved Examples from the Chapter: Chemistry in Everyday Life
Example 1. Which site of an enzyme is known as an allosteric site?
Solution: The allosteric site is the site that is not the active site as some of the drugs bind to an enzyme. As a result, the structure of the enzyme changes, making it difficult for the substrate to recognise the active site.
Key points to remember: Need to remember the enzyme catalysis and binding of the receptor to the particular site.
Example 2. What type of forces are involved in binding a substrate to the active site of an enzyme?
Solution: Substrates that bind to the enzyme’s active site through a variety of interactions. These forces are mainly Van der Waals, ionic bonding, hydrogen bonding, or dipole-dipole interaction.
Key points to remember: Mechanism of the enzyme catalysis, type of the interactions between the receptor and the enzyme.
Solved Questions from the Previous Years’ Question Papers
Question 1: Aspirin is known as
(1) Acetyl salicylic acid
(2) Phenyl salicylate
(3) Acetyl salicylate
(4) Methyl salicylic acid
Solution: Aspirin is called Acetyl salicylic acid.
Therefore, option (1) is the answer.
Question 2: Which of the following is/are a bactericidal antibiotic?
(1) Ofloxacin
(2) Tetracycline
(3) Chloramphenicol
(4) Erythromycin
Solution: Ofloxacin is a bactericidal antibiotic.
Therefore, option (1) is the answer.
Question 3: Which of the following are called the neurologically inactive drugs?
1) Analgesics
2) Barbiturates
3) Antipyretics
4) Antihistamines
Solution: Antihistamines are neurologically inactive drugs.
Therefore, option (4) is the correct answer.
Practice Questions
Question 1: Which of the following is not an analgesic?
1) Aspirin
2) Paracetamol
3) Morphine
4) Salvarsan
Answer: (4) Salvarsan.
Question 2: Which of the following is a narrow spectrum antibiotic?
1) Amoxycillin
2) Chloramphenicol
3) Vancomycin
4) Dysidazirine
Answer: (4) Dysidazirine
Question 3: Which of the following are not supposed to treat humans directly?
1) Disinfectants
2) Antimalarials
3) Antiseptics
4) Antibiotics
Answer: (1) Disinfectants.
Conclusion
Chemistry is primarily concerned with the study of materials and the creation of new materials for the benefit of humanity. Medication is a substance that influences human metabolism and helps people recover from illness. These can be harmful if taken in larger dosages than suggested. Chemotherapy is the use of chemicals for medicinal purposes. Biological macromolecules such as carbohydrates, proteins, lipids, and nucleic acids are commonly interacting with drugs. These molecules are referred to as target molecules. Drugs are developed to interact with certain targets in the least amount of time possible so that they do not influence other targets.
This reduces negative effects and concentrates the drug's efficacy. Drug chemistry is concerned with stopping or killing germs, keeping the body from contracting numerous infectious illnesses, and relieving mental stress, among other things. Therefore, the chemistry in everyday life examples are drugs, food additives, cleaning agents, etc. These chemicals show the importance of chemistry in everyday life.
FAQs on NEET Chapter - Chemistry in Everyday Life
1. How does chemistry affect day to day life?
Chemical applications in the industry have a direct impact on our everyday lives, including what we eat, clothing, travel, technology, how we treat illnesses, and how we receive power, to mention a few. Our understanding of chemistry is continually deepening, and new discoveries are being made.
2. What is the importance of food additives?
Preservatives such as flavours, antioxidants, edible colours, sweeteners, and nutritional supplements are added to make the food appealing, healthy, and consumable. Preservatives also help to keep food from spoiling due to microbial development. Artificial sweeteners are used by diabetics who must avoid sugar in their diet.
3. What is the harmful effect of hyperacidity?
Sudden stomach discomfort is a symptom of hyperacidity. Vomiting, a lack of appetite, and heartburn are all possible side effects. It can also cause persistent indigestion and, in the most severe situations, stomach ulcers.























