
Equilibrium Revision Notes Free PDF for NEET Preparation and Faster Revision
Every chemical reaction reaches its equilibrium state given that conditions such as volume, temperature, and pressure are at optimum levels. There are also other conditions that control the outcome of chemical reactions. In this chapter, students will come to know what chemical equilibrium is and how it can be controlled. They will also learn how to interpret the equilibrium of a chemical reaction using proven principles. To understand these fundamental concepts easily, download and refer to the Equilibrium Class 11 notes for free from Vedantu.
These revision notes are prepared by the subject experts at Vedantu to offer an easy-to-understand explanation of all the concepts related to equilibrium. These notes will help students to revise this chapter better and to recall what they have studied to solve NEET questions.
Note: 👉Explore Your Medical College Options with the NEET Rank and College Predictor 2024.
NEET Chemistry Revision Notes - Chapter Pages
NEET Chemistry Chapter-wise Revision Notes | |
Classification of Elements and Periodicity in Properties Notes | |
Equilibrium Notes | |
Access NEET Revision Notes Chemistry Equilibrium
Equilibrium is the state during a reaction in which there is no change in the temperature, pressure, or concentration of reactants and products with time.
An equilibrium is represented by a double arrow, $\rightleftarrows$, in a chemical reaction.
When equilibrium is attained, the forward reaction rate becomes equal to the reverse reaction rate.
There are two types of equilibriums:
Physical equilibrium and
Chemical equilibrium.
Physical Equilibrium: This equilibrium exists between the two different physical states of the substance. There is no change in the chemical composition of the substances. Example:
Solid-Liquid Equilibrium: ${{\text{H}}_{\text{2}}}{\text{O(s)}} \rightleftarrows {{\text{H}}_{\text{2}}}{\text{O(l)}}$
Liquid-Gas Equilibrium: ${{\text{H}}_{\text{2}}}{\text{O(l)}} \rightleftarrows {{\text{H}}_{\text{2}}}{\text{O(g)}}$
Solid-Gas Equilibrium: ${{\text{I}}_2}{\text{(s)}} \rightleftarrows {\text{ }}{{\text{I}}_2}{\text{ (g)}}$
Solid- Solution equilibrium: ${\text{NaCl (s)}} \rightleftarrows {\text{ NaCl (aq)}}$
Chemical Equilibrium: In this type of equilibrium, the reactants and products are different but their concentration is not changing with time. This is due to the fact that the rate of formation of products (forward reaction) is equal to the rate of formation of reactants (Reverse reaction).
Example: Decomposition of calcium carbonate
${\text{CaCO}}{}_3{\text{(s)}} \rightleftarrows {\text{ CaO(s) + C}}{{\text{O}}_{\text{2}}}{\text{(g)}}$
In this reaction, the rate of decomposition of CaCO3 is equal to the rate of combination of CaO and CO2.
Type of Chemical Equilibriums:
Homogeneous equilibrium: In such reactions, both reactants and products are in the same physical phase. Example: ${{\text{N}}_{\text{2}}}{\text{(g) + 3}}{{\text{H}}_{\text{2}}}{\text{(g) }} \rightleftarrows {\text{2N}}{{\text{H}}_3}{\text{ (g)}}$
Heterogeneous equilibrium: In such reactions, reactants and products are in different physical phases. Example: ${\text{MgO (s) + C}}{{\text{O}}_2}{\text{(g)}} \rightleftarrows {\text{MgC}}{{\text{O}}_3}{\text{ (s)}}$
Dynamic Nature of Equilibrium:
Dynamic equilibrium means that the rate of forward reaction has become equal to the rate of the reverse reaction. Although there is no change is observed in the concentration of reactants and products at equilibrium, it does not mean that the reaction has stopped. The rate at which products are forming has become equal to the rate by which reactants are again changing in the products.
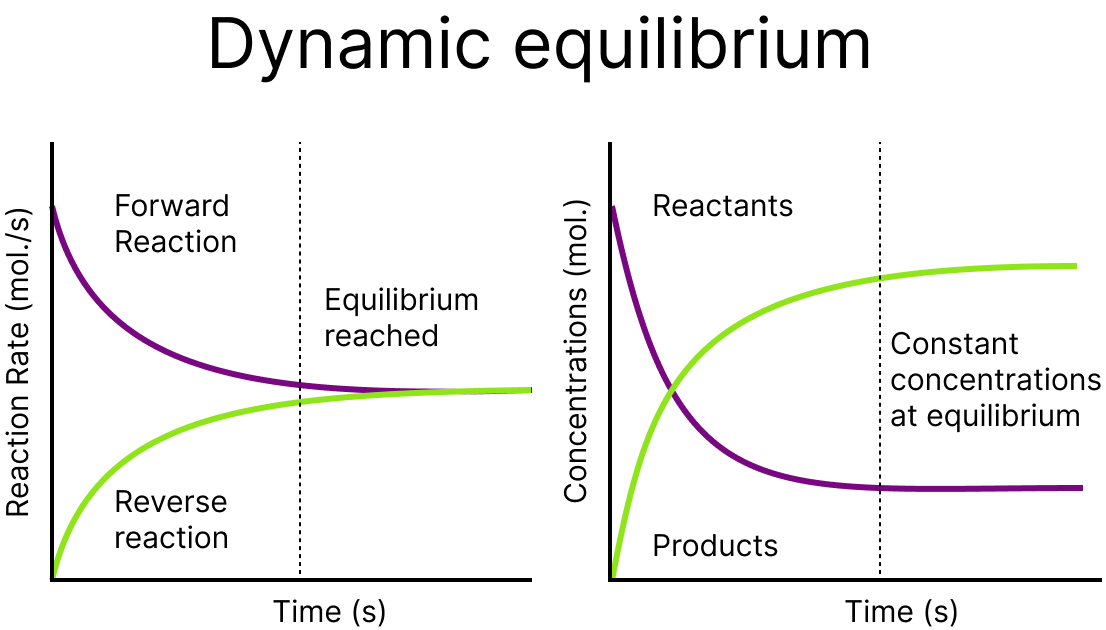
Dynamic Equilibrium
Law of Chemical Equilibrium:
According to this law, the ratio of the concentration of the products to the concentration of the reactants, each term raised to the power of their stoichiometric coefficients, is a constant and this constant is called the equilibrium constant.
For a general reaction: ${\text{aA + bB}} \rightleftarrows {\text{cC + dD}}$
Equilbrium constant is expressed as: ${{\text{K}}_c} = \dfrac{{{{{\text{[C]}}}^{\text{c}}}{\text{.[D}}{{\text{]}}^{\text{d}}}}}{{{{{\text{[A]}}}^{\text{a}}}{\text{.[B}}{{\text{]}}^{\text{b}}}}}$
Equilibrium Constant:
The equilibrium constant shows the relationship between the concentration of reactants and products at equilibrium.
It is derived as:
For the reaction: ${\text{aA + bB}} \rightleftarrows {\text{cC + dD}}$
Rate of the forward reaction, ${{\text{R}}_{\text{f}}}{\text{ = }}{{\text{K}}_{\text{f}}}{{\text{[A]}}^{\text{a}}}{{\text{[B]}}^{\text{b}}}$
Rate of the reverse reaction, ${{\text{R}}_{\text{b}}}{\text{ = }}{{\text{K}}_{\text{b}}}{{\text{[C]}}^{\text{c}}}{{\text{[D]}}^{\text{d}}}$
At equilibrium, rate of forward reaction = rate of reverse reaction.
Therefore,
${{\text{K}}_{\text{f}}}{{\text{[A]}}^{\text{a}}}{{\text{[B]}}^{\text{b}}}{\text{ = }}{{\text{K}}_{\text{b}}}{{\text{[C]}}^{\text{c}}}{{\text{[D]}}^{\text{d}}}$
${{\text{K}}_{\text{c}}}{\text{ = }}\dfrac{{{{\text{K}}_{\text{f}}}}}{{{{\text{K}}_{\text{b}}}}}{\text{ = }}\dfrac{{{{{\text{[C]}}}^{\text{c}}}{{{\text{[D]}}}^{\text{d}}}}}{{{{{\text{[A]}}}^{\text{a}}}{{{\text{[B]}}}^{\text{b}}}}}$
For the reverse reaction, the value of Kc is the inverse of the equilibrium constant for the forward reaction.
${{\text{K}}_{\text{f}}}{\text{ = }}\dfrac{{\text{1}}}{{{{\text{K}}_{\text{b}}}}}$
When the equation is multiplied by any factor (say n), then the equilibrium constant for the new equation is: ${K_c}^{'}=K_c\cdot n$
Reaction Quotient (Q): At any point other than equilibrium during a reaction, the ratio of the concentration of products to the concentration of reactants, each term raised to the power of their stoichiometric coefficients, is known as reaction quotient.
When Q = Kc, then the reaction is at equilibrium.
When Kc >Q, then the reaction proceeds in the forward direction. More products are formed.
When Kc <Q, then the reaction proceeds in the reverse direction. More reactants are formed.
Le-Chatelier’s Principle:
According to Le-Chatelier’s principle, if any change is made to equilibrium then the position of equilibrium shifts in order to counteract that change to re-establish the equilibrium.
Effect of Change in Temperature:
For exothermic reactions, when the temperature of the system is increased, more heat is added to the reaction system, thus to counteract this rise in heat, the equilibrium will go in the reverse direction to absorb this extra heat and vice-versa.
For endothermic reactions, when the temperature of the system is increased, the forward reaction is favored and vice-versa.
Effect of Change in Pressure:
If the pressure of the system is increased, the equilibrium will go in the direction which decreases the pressure to counteract this increase. Thus, the reaction will go towards lesser gaseous molecules and vice-versa.
Effect of Change in Concentration:
When the concentration of reactants increases, the reaction favors the forward reaction. And when the concentration of the products increases, the reaction favors the reverse reaction.
Effect of Addition of Inert Gas:
At constant volume, there is no effect on the Equilibrium. At constant pressure, equilibrium shifts towards the greater number of gaseous molecules. There is no effect of the addition of a catalyst on the equilibrium.
Thermodynamics of Chemical Equilibrium:
The equation that relates G (Gibbs free energy change) and the Keq (equilibrium constant) of a reaction is:
${{\Delta G = \Delta }}{{\text{G}}^{\text{o}}}{\text{ + RTln}}{{\text{K}}_{{\text{eq}}}}$
Where
Go is the standard Gibbs free energy change
R: Universal gas constant
T: Temperature
When the reaction is at equilibrium, the value of G becomes zero.
Thus,
${{\Delta G = 0 = \Delta }}{{\text{G}}^{\text{o}}}{\text{ + RTln}}{{\text{K}}_{{\text{eq}}}}{\text{ }}$
$\therefore {{\Delta }}{{\text{G}}^{\text{o}}} = - {\text{RTln}}{{\text{K}}_{{\text{eq}}}}{\text{ }} = - {\text{2}}{\text{.303RTlog}}{{\text{K}}_{{\text{eq}}}}$
Relation between Keq (equilibrium constant) and ∆H° (Standard enthalpy change) and ∆S° (Standard entropy change)-
${{log }}{{\text{K}}_{{\text{eq}}}} = \dfrac{{{{ - \Delta }}{{\text{H}}^{\text{o}}}}}{{{\text{2}}{\text{.303RT}}}}{\text{ + }}\dfrac{{{{\Delta }}{{\text{S}}^{\text{o}}}}}{{{\text{2}}{\text{.303RT}}}}$
Ionic Equilibrium
An ionic equilibrium is established between an ionic compound and its dissociated ions in the solution.
Example: ${{\text{X}}_{\text{a}}}{{\text{Y}}_{\text{b}}} \rightleftarrows {\text{a}}{{\text{X}}^{\text{ + }}}{\text{ + b}}{{\text{Y}}^{\text{ - }}}$
The substances that dissociate into their constituent ions when dissolved in solution and also conduct electricity are referred as electrolytes. They include acids, bases and salts.
Electrolytes Can be Divided Into Two Categories:
Strong electrolytes and
Weak electrolytes.
Strong Electrolytes:
They completely ionize in their solution. Strong acids (HCl, HBr, HI, HNO3, H2SO4), strong bases, and water-soluble salts like NaCl are strong electrolytes.
Weak Electrolytes:
They ionize partially when dissolved. Weak acids, weak bases and insoluble salts like AgCl.
Ostwald Dilution Law:
According to this law, the degree of dissection (α) of any weak electrolyte and equilibrium constant are related as-
${\text{K = }}\dfrac{{{\text{C}}{{{\alpha }}^{\text{2}}}}}{{(1 - {{\alpha }})}}$
For weak electrolytes, since $\alpha {\text{ < < < 1, thus (1 - }}\alpha ) = 1$
$\therefore {\text{K = C}}{{{\alpha }}^{\text{2}}}{{ or \alpha = }}\sqrt {{\text{K/C}}} $
Next, the dissociation constant of an acid is represented by Ka and the dissociation constant of a base is represented by Kb. The relationship between Ka and Kb is:
${{\text{K}}_{\text{a}}}{{ \times }}{{\text{K}}_{\text{b}}}{\text{ = }}{{\text{K}}_{\text{w}}}{\text{ = 1}}{{.0 \times 1}}{{\text{0}}^{{\text{ - 14}}}}$
Where Kw is the ion-product constant of liquid water.
The acid strength is defined by its pH.
${\text{pH = - log [}}{{\text{H}}^ + }]$
For a weak dibasic acid: ${\text{pH = }}\dfrac{{{\text{(p}}{{\text{K}}_{{{\text{a}}_{\text{1}}}}}{\text{ + p}}{{\text{K}}_{{{\text{a}}_{\text{2}}}}}{\text{)}}}}{2}$ where ${{\text{K}}_{{{\text{a}}_{\text{1}}}}}$ is the first dissociation constant of the acid and ${{\text{K}}_{{{\text{a}}_2}}}$ is the second dissociation constant of the acid.
The strength of a base is defined by its pOH.
${\text{pOH = - log[O}}{{\text{H}}^{\text{ - }}}{\text{]}}$
The relation between pH and pOH is: ${\text{pH + pOH = 14}}$
Common ion Effect: When equilibrium is established between an ionic compound and its ions, then adding a common ion will shift the direction of the equilibrium in order to consume that ion as per the Le-Chatelier’s principle.
Salts: Salts are produced when an acid reacts with a base.
Ionization of Salts: When salts ionize in a solution, their ions further react with water forming either an acidic, a basic, or a neutral solution.
Salt Formed From | Salt Solution |
Strong acid + Strong base | Neutral |
Weak acid + Strong base | Basic |
Strong acid + Weak base | Acidic |
Buffer Solution
Buffer solutions are those that resist the change in their pH on small addition of acid or base. They are formed by mixing a weak acid and its strong conjugate base or a weak base and its strong conjugate acid.
Henderson-Hasselbalch Equation: The pH of the buffer solution is to be determined using the Henderson-Hasselbalch equation which is expressed as:
${\text{pH = p}}{{\text{K}}_{\text{a}}}{\text{ + log}}\dfrac{{{\text{[}}{{\text{A}}^{\text{ - }}}{\text{]}}}}{{{\text{[HA]}}}}$
Where,
[A-] is the concentration of the conjugate base
[HA] is the concentration of the acid.
When a basic buffer (Weak base + conjugate acid) is given, pOH is calculated using Henderson-Hasselbalch equation as-
${\text{pOH = p}}{{\text{K}}_{\text{b}}}{\text{ + log}}\dfrac{{{\text{[BH]}}}}{{{\text{[}}{{\text{B}}^ - }{\text{]}}}}$
Where,
[B-] is the concentration of the base
[BH] is the concentration of the conjugate acid.
Solubility and Solubility Product
The solubility of a substance is defined as the maximum amount that can be dissolved in a solvent at a specific temperature.
For an ionic compound XY which dissociates in water as: ${\text{XY}} \rightleftarrows {{\text{X}}^{\text{ + }}}{\text{ + }}{{\text{Y}}^{\text{ - }}}$
The solubility product (Ksp) is written as: ${{\text{K}}_{{\text{sp}}}}{\text{ = [}}{{\text{X}}^{\text{ + }}}{\text{][}}{{\text{Y}}^{\text{ - }}}{\text{]}}$
Ionic product (I.P.): The product of the concentration of ions present in the solution.
When I.P. < Ksp, it means the solution is unsaturated.
When I.P. > Ksp, it means the solution is supersaturated and precipitate will form.
When I.P. = Ksp, it means the solution is saturated and no precipitate formation will occur.
Importance of Class 11 Chemistry Chapter Equilibrium
This chapter holds immense importance as it explains the mechanism of chemical reactions including the various factors affecting its outcomes. This fundamental chapter explains how every reaction has two directions to proceed and how the conditions like temperature, pressure, etc., can control the direction of completion of a reaction.
According to this chapter, a chemical reaction has physical and chemical equilibrium. It will describe the different types of physical equilibrium with examples. The chemical equilibrium will also be explained using examples. The classification of chemical equilibrium explains the concept of reversible, irreversible, homogenous, and heterogeneous equilibriums following the concentration of reactants and products.
Every type of equilibrium will be explained using mathematical expressions so that students can find out how to calculate the outcomes using proper units. This chapter will describe how the changes in the factors controlling a chemical reaction will influence the concentration of products and the other outcomes.
The laws related to chemical and physical equilibrium are covered with respective mathematical expressions. These notes cover a detailed explanation of laws, principles, and fundamental concepts that will enable students to develop a foundation to solve NEET questions accurately. Students can refer to the Equilibrium Chemistry Class 11 notes for their exam preparation.
Benefits of Equilibrium Class 11 Notes for NEET
Our subject experts have prepared these notes keeping the prime objective of guiding NEET aspirants in their chemistry preparation. These notes will enable students with concise study materials for all definitions, descriptions, and derivations of scientific terms, principles, and laws explained in this chapter.
The concise notes will help students to revise the fundamental concepts of this chapter in a short time, as they get expert-curated notes right at their fingertips.
Revising this chapter faster will help you to complete preparing the entire syllabus of NEET Chemistry easily. They will also be able to use their time efficiently and proceed to study another chapter.
Download Free Equilibrium NEET Notes PDF
You can now avail of the Equilibrium Class 11 notes Chemistry PDF download for free from Vedantu at your convenience. Use these notes to prepare the topics covered in this chapter more efficiently. Learning and revising the Class 11 Chemistry Equilibrium revision notes will help students to prepare all important topics of this chapter.
Other Important Links Related to NEET Equilibrium
Other Important Links for NEET Equilibrium |
FAQs on Equilibrium Class 11 Notes for NEET Chemistry Revision [Free PDF Download]
1. What is a catalyst?
A substance that promotes a reaction in a particular direction without participating in the reaction is called a catalyst. Example: Platinum is used as a catalyst to manufacture sulphuric acid.
2. What happens when there is a change in temperature?
The change in temperature influences the outcome of endothermic and exothermic reactions. Increasing temperature pushes a chemical reaction to its endothermic direction and vice versa.
3. What is a strong acid?
The acid that produces a higher concentration of hydrogen ions (H+) ions in a solution is called a strong acid. It dissociates completely in its aqueous solution. Example – Hydrochloric acid (HCl).
4. What is a weak acid?
A weak acid partially dissociates in its aqueous solution to release a low amount of hydrogen ions (H+). Example – Acetic acid (CH3COOH) and other organic acids.
























