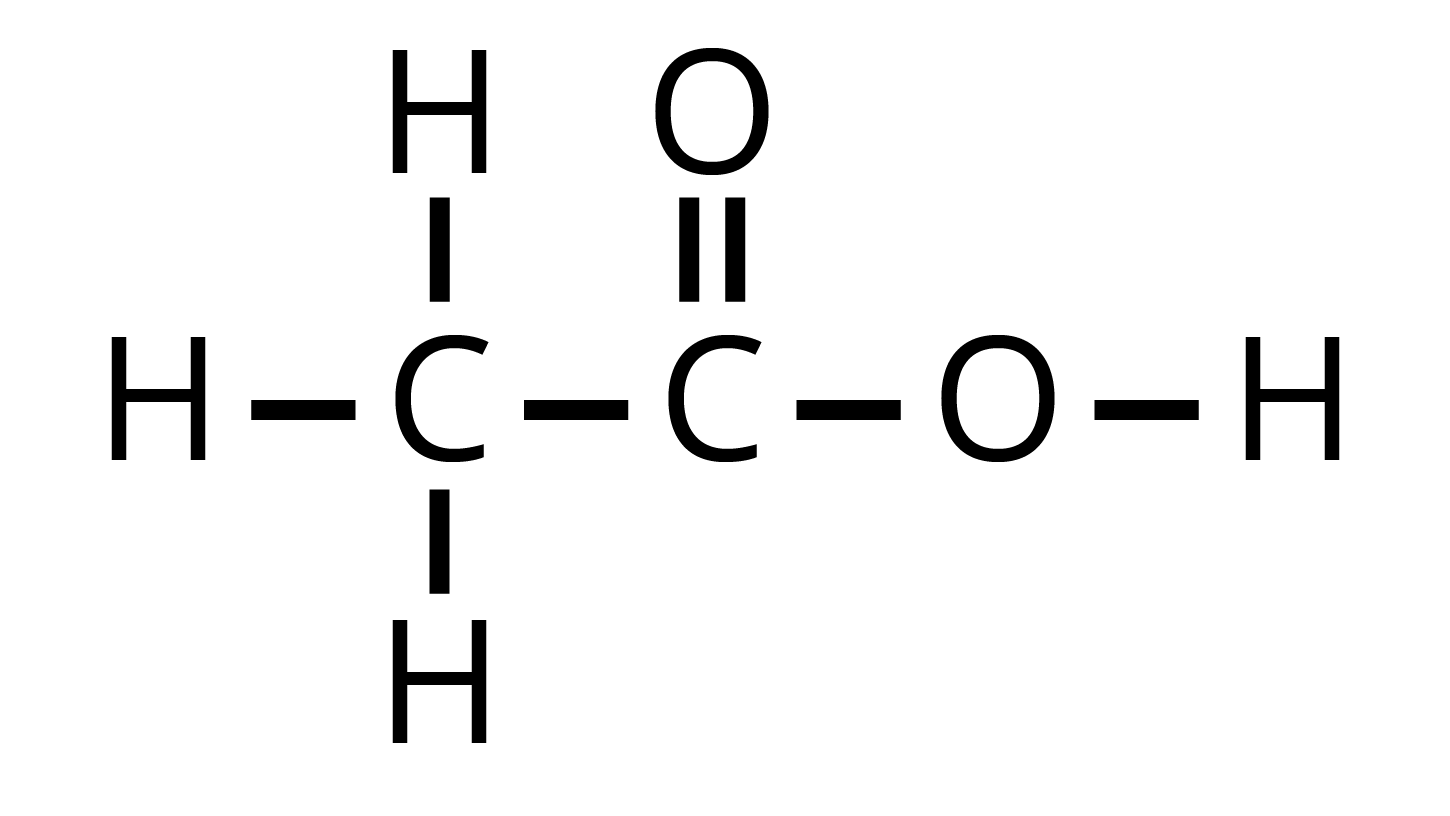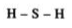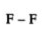Solved NCERT Questions For Class 10 Science Chapter 4 In Hindi - Free PDF
NCERT Solutions For Class 10 Science In Hindi Chapter 4 Carbon And Its Compounds - 2025-26
FAQs on NCERT Solutions For Class 10 Science In Hindi Chapter 4 Carbon And Its Compounds - 2025-26
1. What are the two properties of carbon which lead to the huge number of carbon compounds we see around us?
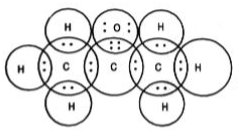
We already know that carbon is the core of every matter. Everything, when burnt, turns into carbon. Carbon reacts with other elements to form new substances recognized as carbon compounds. Two reasons for the formation of carbon compounds are:
Carbon atoms form covalent bonds with other atoms due to having tetra valency.
Each carbon atom can form not just a single bond, but also double and triple bonds with other atoms.
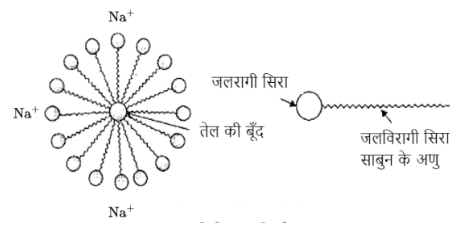
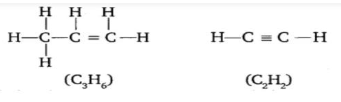
2. A mixture of oxygen and ethyne is burnt for welding. Can you tell why a mixture of ethyne and air is not used?
Wielding is the process used to join metals or other materials using high temperature. High heat is the key point here because metals usually have high melting points. A mixture of ethyne and air is not used for welding for the following three reasons:
The mixture does not burn completely.
It leaves a sooty stain behind while burning.
A high temperature cannot be achieved whereas when using oxygen the temperature can be as high as 3000℃.
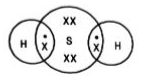
3. What are covalent bonds in Chapter 4 of Class 10 Science?
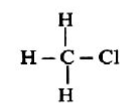
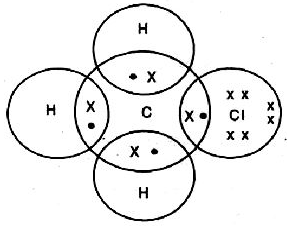
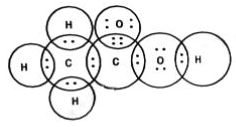
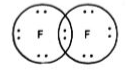
Chemical bonds are of various categories, one of them being a covalent bond. A covalent bond is formed by sharing one or more electrons between two atoms.
They have three types:
Single Covalent Bond - When the atoms share one electron. It is represented by a single line.
Double Covalent Bond - Two electrons are shared between the atoms and this bond is represented by a double line.
Triple covalent bond- Two atoms share three electrons. It is represented by triple lines.
4. Where can I find Class 10 Science Chapter 4: Carbon and Its Compounds NCERT Solutions in Hindi?
Vedantu provides you with detailed NCERT Solutions in English and Hindi. Visit the page-NCERT Solutions of Class 10 Science Chapter 4 to download the solutions free of cost in Hindi. These solutions are all your study materials in one place for thorough exam preparations. They are designed by subject experts especially for the students, hence, they are reliable. 10th Standard is a challenging class for the students and it is beneficial if they gather the study materials beforehand so the preparation and revision become an easy sail.
5. What is isomerism in Chapter 4 of Class 10 Science?
When two or more compounds have the same chemical formula but different chemical structures it is known as isomerism. The compounds that show isomerism are known as isomers. This phenomenon is further categorized into different types based on their properties. Carbon and its Compounds is a vast chapter that students often state to be tough but with the right guidance, you can learn it efficiently. You can get detailed answers about isomerism on NCERT Solutions for Class 10 Science Chapter 4 available on Vedantu website and app.




















 Watch Video
Watch Video