
Which one of the following is a correct set concerning molecule, hybridization, and shape
A.\[BeC{l_2},{\rm{ }}s{p^2}\], linear
B. \[BeC{l_2},{\rm{ }}s{p^2}\], triangular planar
C. \[BC{l_3},{\rm{ }}s{p^2}\], triangular planar
D.\[BC{l_3},{\rm{ }}s{p^3}\], tetrahedral
Answer
233.1k+ views
Hint: Molecules possess shapes. Small molecules i.e, molecules with a single central atom maintain shapes that can be efficiently predicted.
Beryllium chloride has sp hybridization and linear shape.
Complete step by step solution:Here in this question, we have to find out the
the correct set of molecules, hybridization, and shape.
A.\[BeC{l_2},{\rm{ }}s{p^2}\], linear
Here Be is the central atom.
It has two valence electrons. These electrons combine with the electrons of chlorine atoms and form two Be-Cl bonds. So, there is sp hybridization and linear shape.
So, it is incorrect.
B. \[BeC{l_2},{\rm{ }}s{p^2}\], triangular planar
This is also incorrect as this given molecule has a linear shape.
So, B is incorrect.
C. \[BC{l_3},{\rm{ }}s{p^2}\], triangular planar
Boron is the central atom.
It has three valence electrons. These electrons combine with the electrons of chlorine atoms and form three B-Cl bonds. So, there is {\rm{ }}s{p^2}\] hybridization and trigonal planar shape.
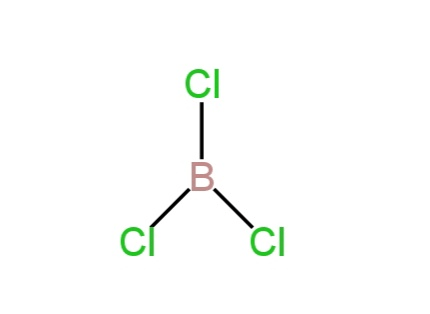
Image: Structure of boron trichloride.
So, C is correct.
D.\[BC{l_3},{\rm{ }}s{p^3}\], tetrahedral
This is incorrect as the given molecule has a planar shape, not a tetrahedral shape.
So, D is incorrect.
So,\[BC{l_3}\] has\[s{p^2}\] and a triangular planar shape.
So, option C is correct.
Note: Boron reacts with halogens to provide the corresponding trihalides. Boron trichloride is yielded industrially by direct chlorination of boron oxide and carbon.
In the laboratory,\[B{F_3}\] reacts with\[AlC{l_3}\] to yield\[BC{l_3}\]
through halogen exchange.
Beryllium chloride has sp hybridization and linear shape.
Complete step by step solution:Here in this question, we have to find out the
the correct set of molecules, hybridization, and shape.
A.\[BeC{l_2},{\rm{ }}s{p^2}\], linear
Here Be is the central atom.
It has two valence electrons. These electrons combine with the electrons of chlorine atoms and form two Be-Cl bonds. So, there is sp hybridization and linear shape.
So, it is incorrect.
B. \[BeC{l_2},{\rm{ }}s{p^2}\], triangular planar
This is also incorrect as this given molecule has a linear shape.
So, B is incorrect.
C. \[BC{l_3},{\rm{ }}s{p^2}\], triangular planar
Boron is the central atom.
It has three valence electrons. These electrons combine with the electrons of chlorine atoms and form three B-Cl bonds. So, there is {\rm{ }}s{p^2}\] hybridization and trigonal planar shape.

Image: Structure of boron trichloride.
So, C is correct.
D.\[BC{l_3},{\rm{ }}s{p^3}\], tetrahedral
This is incorrect as the given molecule has a planar shape, not a tetrahedral shape.
So, D is incorrect.
So,\[BC{l_3}\] has\[s{p^2}\] and a triangular planar shape.
So, option C is correct.
Note: Boron reacts with halogens to provide the corresponding trihalides. Boron trichloride is yielded industrially by direct chlorination of boron oxide and carbon.
In the laboratory,\[B{F_3}\] reacts with\[AlC{l_3}\] to yield\[BC{l_3}\]
through halogen exchange.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




