
Which one of the following complexes shows optical isomerism?
A. $cis[Co{{(en)}_{2}}C{{l}_{2}}]Cl$
B. $trans[Co{{(en)}_{2}}C{{l}_{2}}]Cl$
C. $[Co{{(N{{H}_{3}})}_{4}}C{{l}_{2}}]Cl$
D. $[Co{{(N{{H}_{3}})}_{3}}C{{l}_{3}}]$
($en$ = ethylenediamine)
Answer
232.8k+ views
Hint: Think about what the term optical isomerism means and how a molecule is considered to have optical isomers. Look for a plane of symmetry in all the given molecules.
Complete step by step solution:
Complexes that show optical isomerism exist in two forms, the dextro-rotatory form and the laevo-rotatory form. If plane-polarized light is rotated clockwise after passing through a compound and coming towards you, the compound is considered to have dextro-rotatory molecules. On the other hand, if the plane-polarized light is rotated anticlockwise while moving towards you, then the molecules in the compound are considered to be laevo-rotatory. Whether a molecule will show optical isomerism or not will depend on whether it has a plane of symmetry or not. If a molecule has a plane of symmetry, it does not show optical isomerism. We will check the molecules of each of the given compounds one by one to see whether they are optically active or not.
- $cis[Co{{(en)}_{2}}C{{l}_{2}}]Cl$
The structure of this molecule is:
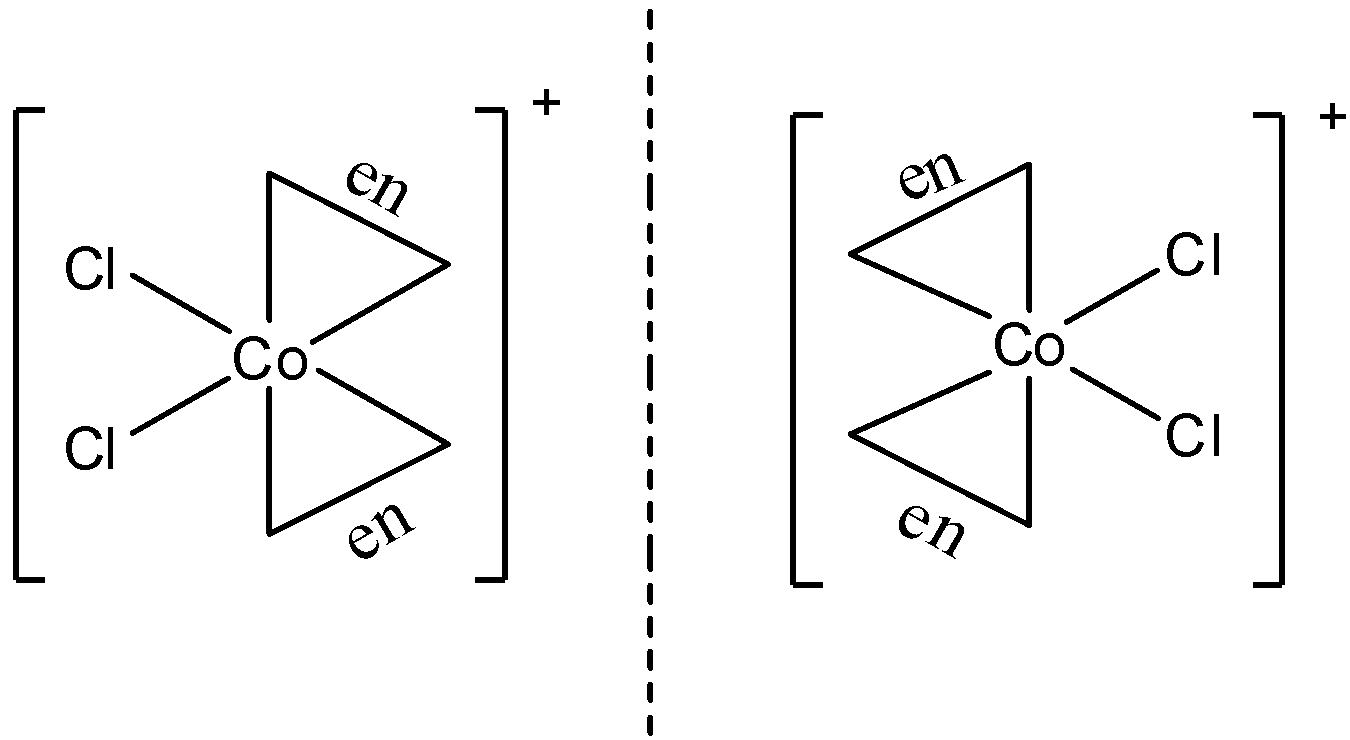
This structure has optical isomers since it does not have a plane of symmetry. Hence, it shows optical isomerism.
- $trans[Co{{(en)}_{2}}C{{l}_{2}}]Cl$
The structure of this molecule is:
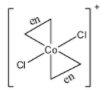
This molecule has a plane of symmetry and thus does not show optical isomerism.
- $[Co{{(N{{H}_{3}})}_{4}}C{{l}_{2}}]Cl$
The structure of this molecule is:
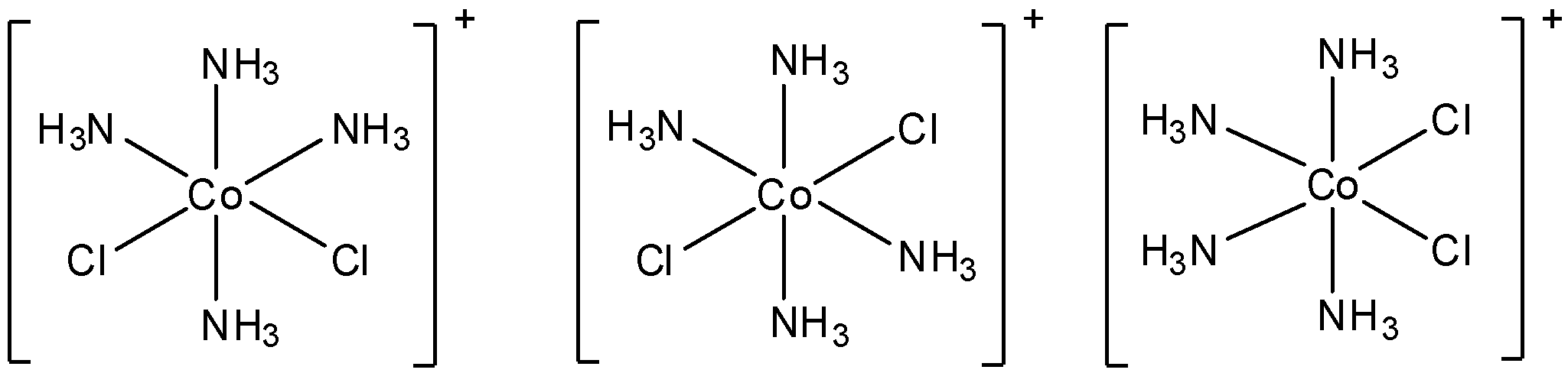
We can see that no matter how we arrange the molecules, there always exists a plane of symmetry. So, this molecule does not show optical isomerism.
- $[Co{{(N{{H}_{3}})}_{3}}C{{l}_{3}}]$
The structure of this molecule is:
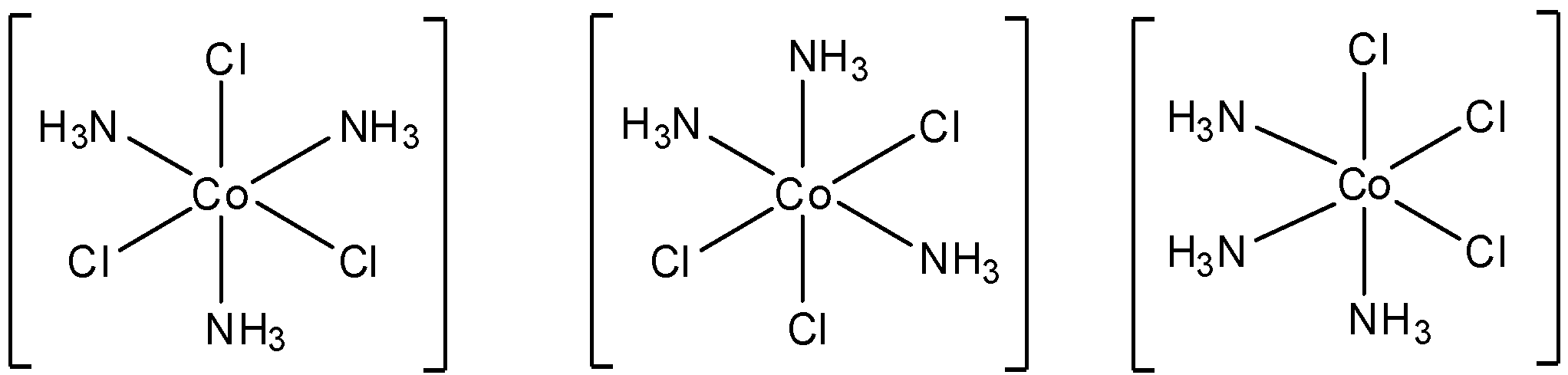
Here too, we can see that a plane of symmetry is present in all the structures that are possible. So, this molecule is not optically active.
Hence, the correct answer to this question is ‘A. $cis[Co{{(en)}_{2}}C{{l}_{2}}]Cl$’.
Note: To determine whether there is a plane of symmetry or not, check for any irrotational bonds or ligands. Double bonds and triple bonds are usually considered to be irrotational and bidentate, tridentate, or polydentate ligands are considered to be irrotational. We can determine whether a molecule is optically active or not based on the presence of irrotational bonds.
Complete step by step solution:
Complexes that show optical isomerism exist in two forms, the dextro-rotatory form and the laevo-rotatory form. If plane-polarized light is rotated clockwise after passing through a compound and coming towards you, the compound is considered to have dextro-rotatory molecules. On the other hand, if the plane-polarized light is rotated anticlockwise while moving towards you, then the molecules in the compound are considered to be laevo-rotatory. Whether a molecule will show optical isomerism or not will depend on whether it has a plane of symmetry or not. If a molecule has a plane of symmetry, it does not show optical isomerism. We will check the molecules of each of the given compounds one by one to see whether they are optically active or not.
- $cis[Co{{(en)}_{2}}C{{l}_{2}}]Cl$
The structure of this molecule is:

This structure has optical isomers since it does not have a plane of symmetry. Hence, it shows optical isomerism.
- $trans[Co{{(en)}_{2}}C{{l}_{2}}]Cl$
The structure of this molecule is:

This molecule has a plane of symmetry and thus does not show optical isomerism.
- $[Co{{(N{{H}_{3}})}_{4}}C{{l}_{2}}]Cl$
The structure of this molecule is:

We can see that no matter how we arrange the molecules, there always exists a plane of symmetry. So, this molecule does not show optical isomerism.
- $[Co{{(N{{H}_{3}})}_{3}}C{{l}_{3}}]$
The structure of this molecule is:

Here too, we can see that a plane of symmetry is present in all the structures that are possible. So, this molecule is not optically active.
Hence, the correct answer to this question is ‘A. $cis[Co{{(en)}_{2}}C{{l}_{2}}]Cl$’.
Note: To determine whether there is a plane of symmetry or not, check for any irrotational bonds or ligands. Double bonds and triple bonds are usually considered to be irrotational and bidentate, tridentate, or polydentate ligands are considered to be irrotational. We can determine whether a molecule is optically active or not based on the presence of irrotational bonds.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




