
Which one among the following represents an amide
Answer
232.8k+ views
Hint: Amides are of two types one is acetamide and the other is benzamide. Both types of amide contains a functional group, amide group or carboxamide which consists of the carbonyl group (carbon bonded with oxygen through double bond) which in turn bonded with an amino group such as -CONR’R”. Acetamide is one in which the amide group is bonded with the alkyl group (like \[-C{{H}_{3}}\] or simple chain) whereas benzamide is one in which the functional group, the amide is bonded with the benzene group (like \[-{{C}_{6}}{{H}_{5}}\] or cyclic compound). Amide’s general formula is RCONR’R” where R can be alkyl or can be benzene (any cyclic compound).
Complete step by step solution:
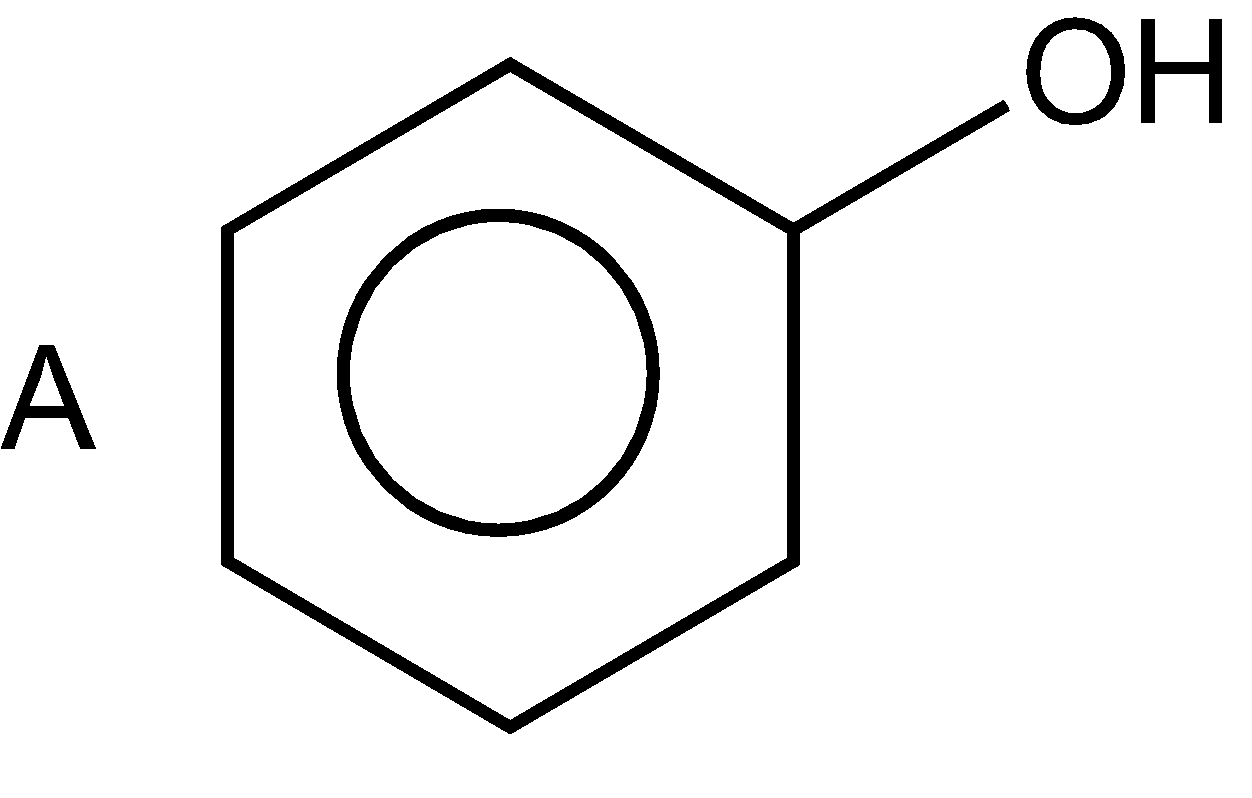
According to IUPAC naming, name of substituent is firstly written (\[-C{{H}_{3}}\] is named as methyl), then the naming of the longest chain of hydrocarbon is written (word root) and at the end naming of the functional group. In the given compound in first option, there is no substituent, and there are six carbon in the ring (cyclohex)ring with three double bonds (at alternative position) which is dispersed in the whole ring (1,3,5-tri-one) so, name of compound is cyclo hex 1,3,5-tri-ene and OH is a functional group whose name is written as “ol” for alcohol (as per IUPAC naming) on replacing ‘e’ of ane (ane and yne). So, the name of the compound is cyclohex-1,3,5-trienol or benzenol and the common name is phenol such as
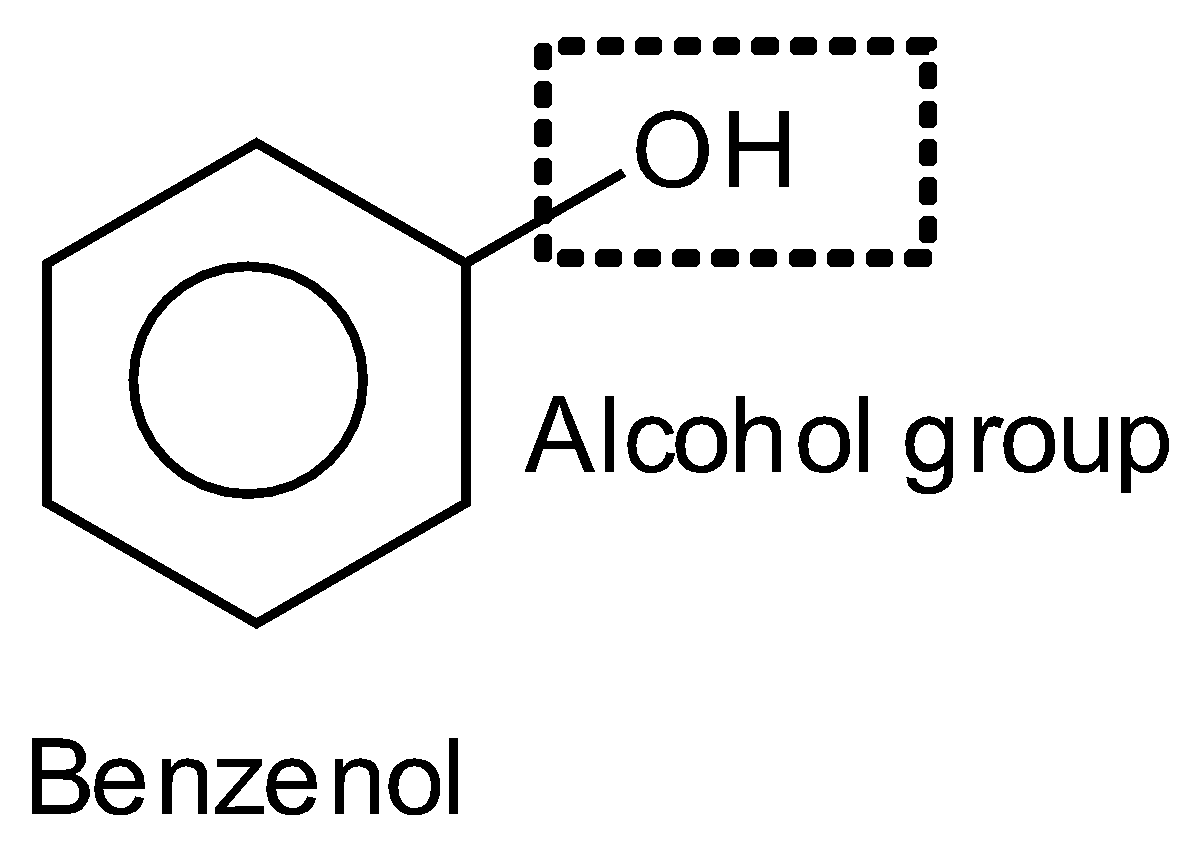
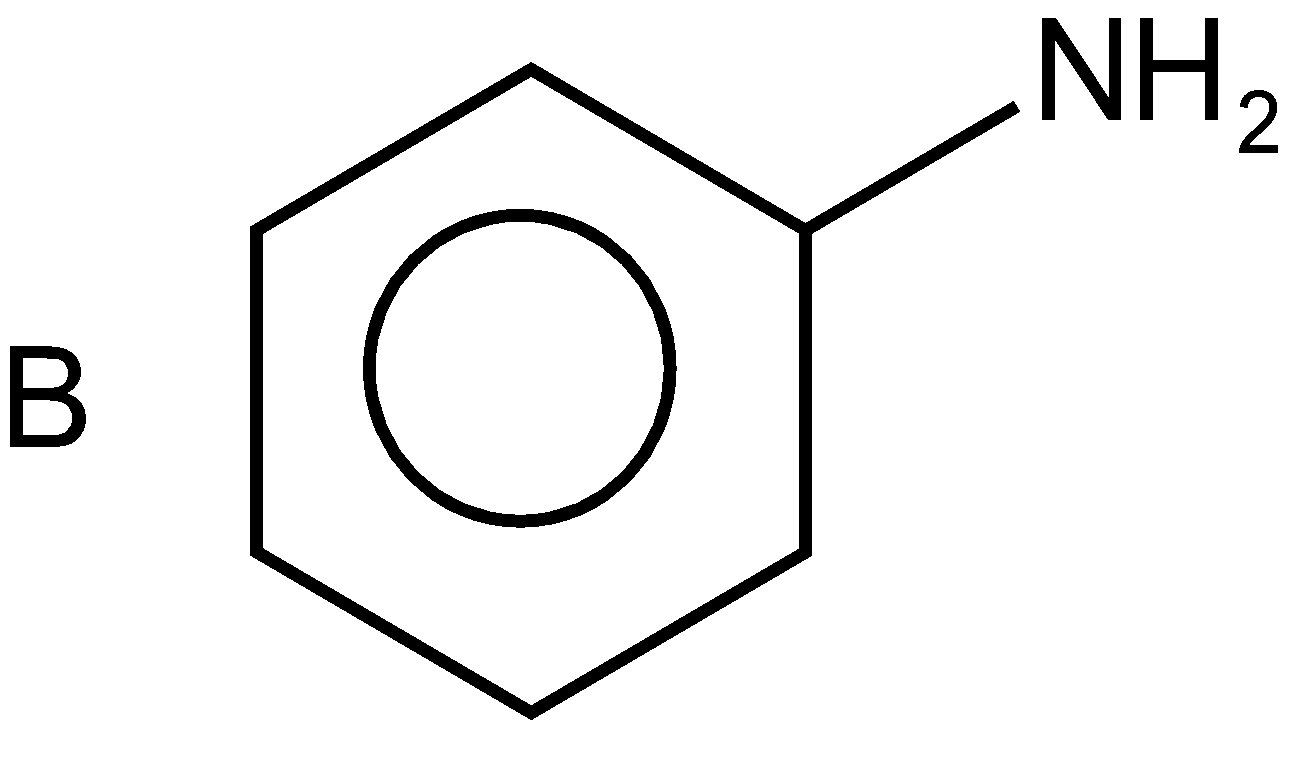
In the second option, the given compound is again formed with the same ring of six carbon and three double bonds on the alternative position which is dispersed to the whole ring. At the ring, the amino group is attached \[-N{{H}_{2}}\]. The common name of this compound is aniline and the IUPAC name is phenylamine where phenyl represents the benzene ring and amine represents the amino group such as
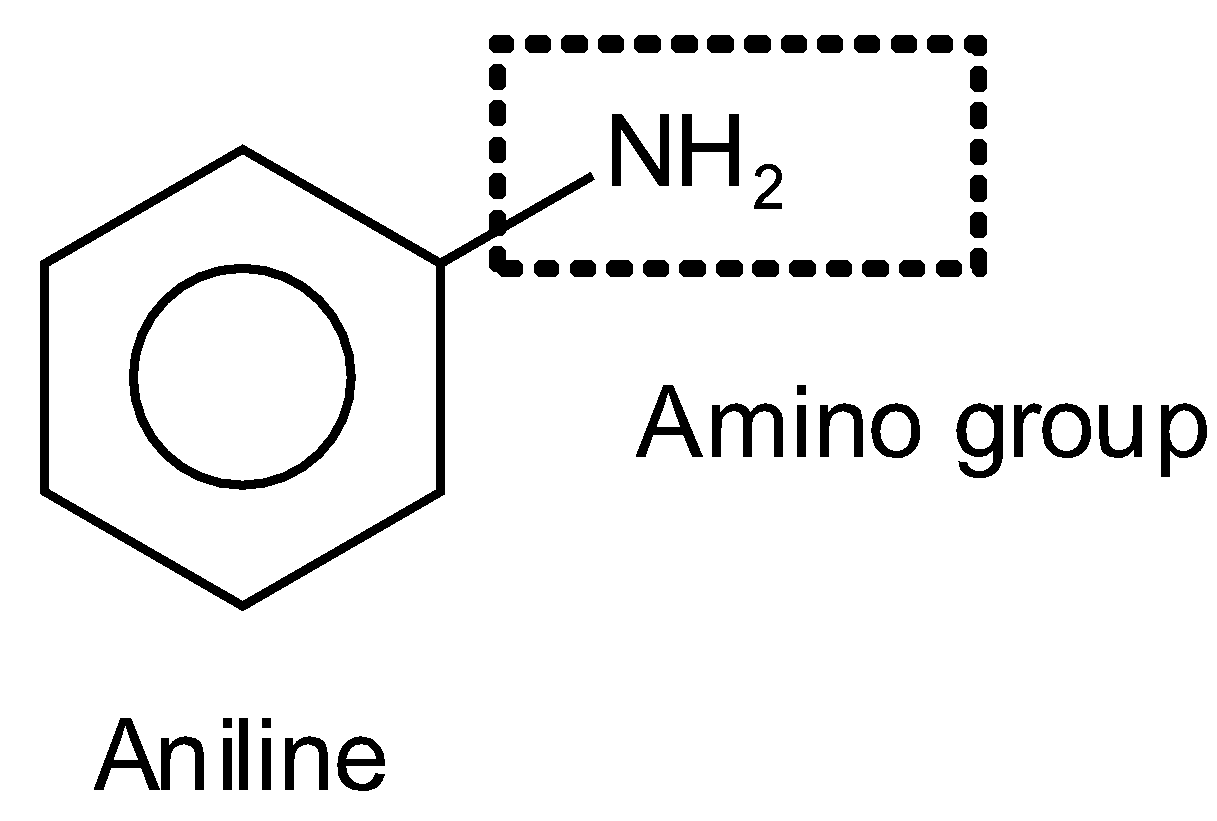
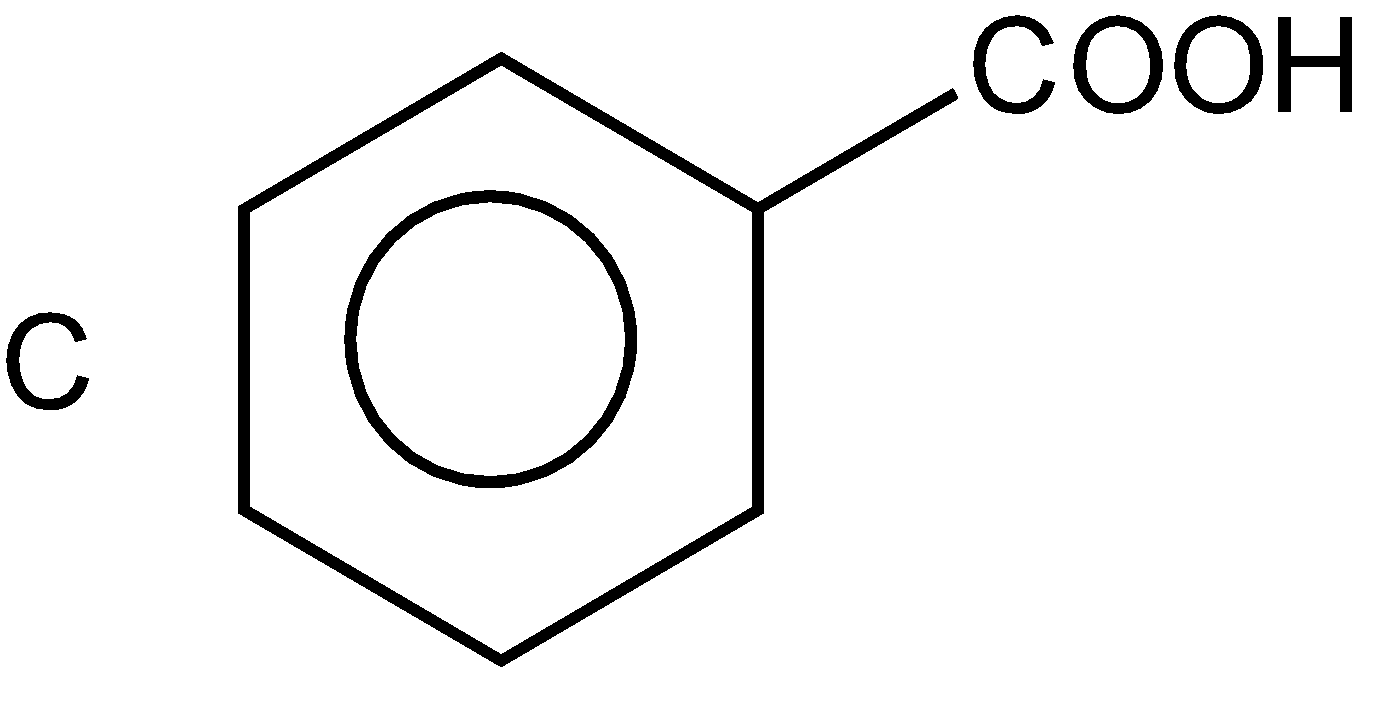
In the fourth option again a benzene ring (hex 1,3,5-triene) is present at which the COOH (carboxylic group) group is attached and it is a functional group, “oic” is used for it on the place of ‘e’ of ene such as benzoic such as

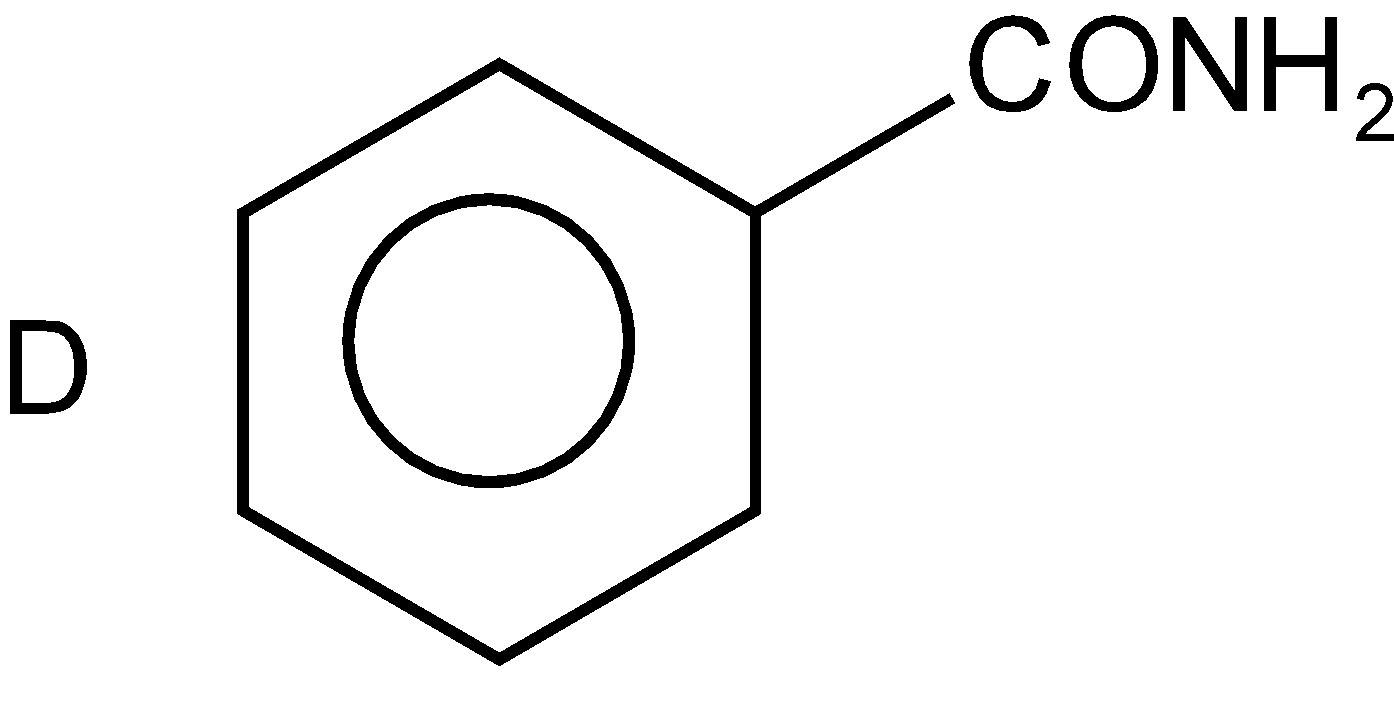
The fourth compound also contains a benzene ring and an amide group (\[CON{{H}_{2}}\]). And its name is Benzamide. And it is of type RCONR’R” where R is the benzene ring and R’ and R” both are hydrogens such as
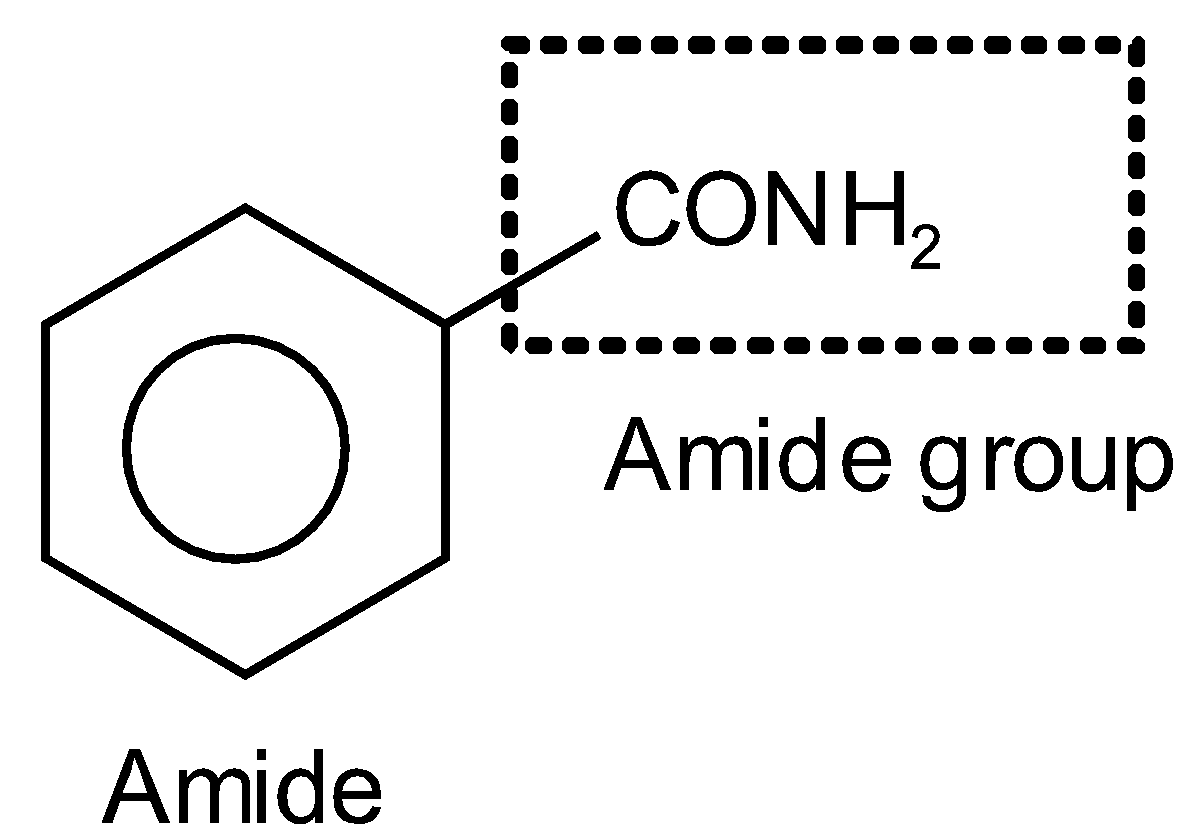
Thus, the correct option is D.
Note: According to IUPAC naming any compound is named following series as Prefix Word root + Suffix. The prefix is substituent like methyl, \[-C{{H}_{3}}\] (when methane \[C{{H}_{4}}\]replace by methyl \[-C{{H}_{3}}\]then “ane” of methane replace by ‘yl’). For naming any compound we need to follow the pattern such as prefix (substituent) + Word root (meth, eth, prop and so on) + primary suffix (degree of saturation and unsaturation like ane for single bond , ene for double bond and yne for triple bond) +secondary suffix (ol for alcohol group, OH replace e of primary suffix).
Complete step by step solution:
According to IUPAC naming, name of substituent is firstly written (\[-C{{H}_{3}}\] is named as methyl), then the naming of the longest chain of hydrocarbon is written (word root) and at the end naming of the functional group. In the given compound in first option, there is no substituent, and there are six carbon in the ring (cyclohex)ring with three double bonds (at alternative position) which is dispersed in the whole ring (1,3,5-tri-one) so, name of compound is cyclo hex 1,3,5-tri-ene and OH is a functional group whose name is written as “ol” for alcohol (as per IUPAC naming) on replacing ‘e’ of ane (ane and yne). So, the name of the compound is cyclohex-1,3,5-trienol or benzenol and the common name is phenol such as

In the second option, the given compound is again formed with the same ring of six carbon and three double bonds on the alternative position which is dispersed to the whole ring. At the ring, the amino group is attached \[-N{{H}_{2}}\]. The common name of this compound is aniline and the IUPAC name is phenylamine where phenyl represents the benzene ring and amine represents the amino group such as

In the fourth option again a benzene ring (hex 1,3,5-triene) is present at which the COOH (carboxylic group) group is attached and it is a functional group, “oic” is used for it on the place of ‘e’ of ene such as benzoic such as

The fourth compound also contains a benzene ring and an amide group (\[CON{{H}_{2}}\]). And its name is Benzamide. And it is of type RCONR’R” where R is the benzene ring and R’ and R” both are hydrogens such as

Thus, the correct option is D.
Note: According to IUPAC naming any compound is named following series as Prefix Word root + Suffix. The prefix is substituent like methyl, \[-C{{H}_{3}}\] (when methane \[C{{H}_{4}}\]replace by methyl \[-C{{H}_{3}}\]then “ane” of methane replace by ‘yl’). For naming any compound we need to follow the pattern such as prefix (substituent) + Word root (meth, eth, prop and so on) + primary suffix (degree of saturation and unsaturation like ane for single bond , ene for double bond and yne for triple bond) +secondary suffix (ol for alcohol group, OH replace e of primary suffix).
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




