




What is Iodoform Reaction?
The Iodoform or haloform test is usually performed to detect the presence of aldehydes and ketones containing the alpha-methyl group. It is also useful to distinguish ethanol from methanol. The test can be performed using iodine with an aqueous solution of sodium hydroxide (NaOH) or using potassium iodide (KI) with a solution of sodium hypochlorite (NaCIO). Carbonyl compounds with the structure R-CO-CH3 and alcohols with the structure R-CH(OH)CH3 readily undergo the iodoform test.
The chemical reaction in which methyl ketone is oxidised to a carboxylate by reaction with aqueous HO- and I2. The reaction also produces iodoform (CHI3), a yellow solid that can precipitate from the reaction mixture. The iodoform reaction is used as a chemical test for the presence of a methyl ketone moiety. The triiodomethane (iodoform) reaction can be used to determine the presence of CH3CO groups in aldehydes and ketones. Two quite different mixtures of reagents can be used to carry out this reaction.
Iodoform Structure
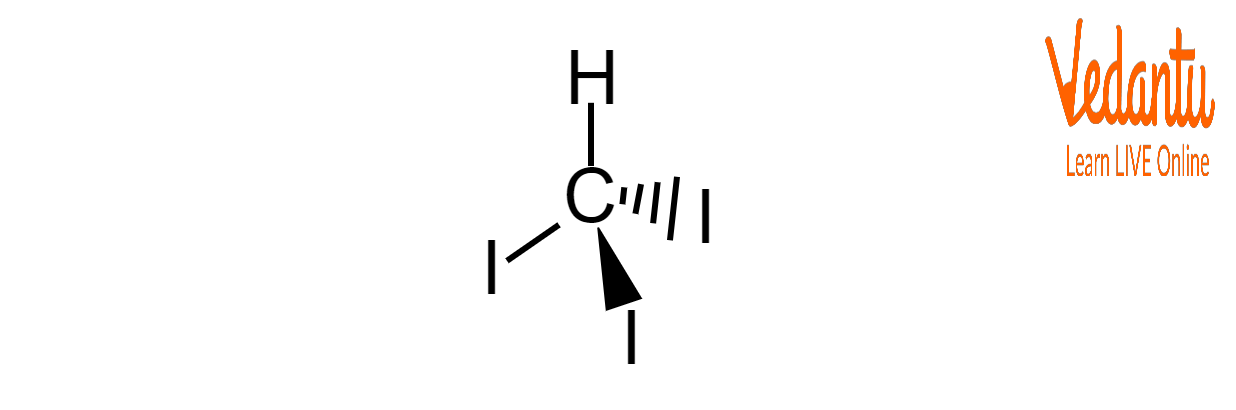
Structure of Iodoform
Iodoform is an organic iodine compound with the formula CHI3 and has a tetrahedral molecular geometry. It is a relatively water-insoluble yellow solid that is chemically reactive in free radical-generating reactions.
Physical Properties of Iodoform
It has a melting point of 121 degrees Celsius.
It is a yellow crystalline solid.
It has a distinct, unpleasant smell.
It is insoluble in water, although soluble in ethyl alcohol and ether.
Chemical Properties of Iodoform
Carbylamine Reaction: when iodoform is ignited with a primary (1) amine (aromatic or aliphatic) and caustic potassium, an isocyanide or a carbylamine equivalent is generated.
Reducing Properties: When reduced with red phosphorus and hydriodic acid, it gives methylene iodide.
Hydrolysis: When heated with potassium hydroxide, alcohol will hydrolyze, releasing formic acid, which interacts with KOH to produce potassium formate.
Reduction: When heated with silver powder, it undergoes a dehalogenation reaction and produces acetylene.
Reaction with Silver Nitrate: Iodoform produces a yellow precipitate of silver iodide when heated with silver nitrate alcohol. In this reaction, it behaves differently from chloroform, not forming a precipitate with silver nitrate.
Stability: When heated, iodoform decomposes to iodine vapour. When exposed to air, moisture, or light, it decomposes. Due to the release of free iodine, it has an antibacterial effect.
Iodoform Test Process
When iodine is added to an unspecified compound containing an aldehyde or a ketone, in the presence of excess sodium hydroxide, a pale yellow precipitate called triiodomethane or iodoform is synthesised with an "antiseptic" odour.
The presence of Aldehydes and Ketones is confirmed due to the precipitation of triiodomethane.
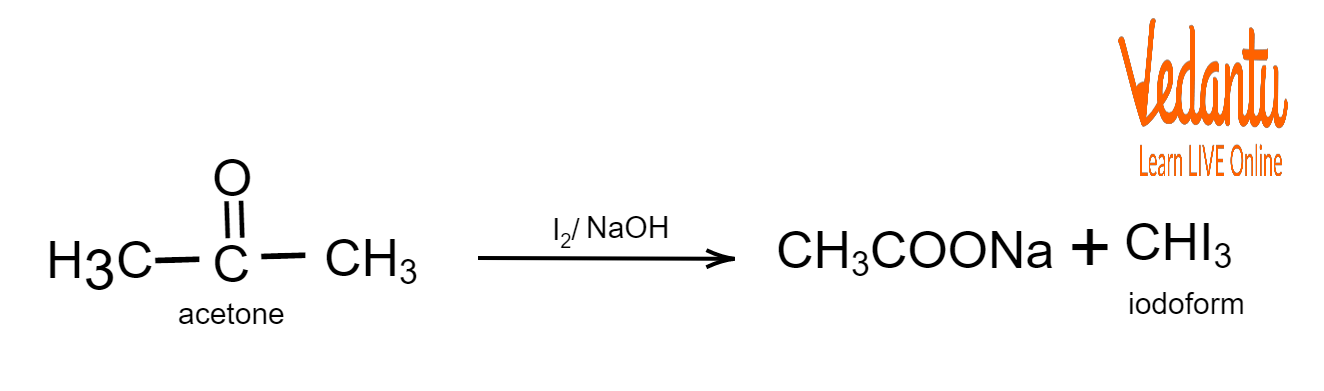
Iodoform Test Reaction
Acetone undergoes the iodoform test by reacting with iodine and aqueous sodium hydroxide (NaOH) to form sodium acetate (CH3COONa) and a yellow precipitate known as iodoform or triiodomethane (CHI3).
The Iodoform test can also be used to distinguish 2-Propanol from 1-Propanol.
Tertiary alcohols did not test positive for iodoform.
Iodoform Reaction Mechanism
The complete mechanism of the iodoform reaction takes place in two steps.
The first step involves replacing the hydrogen atoms in the methyl group with iodine by forming water.
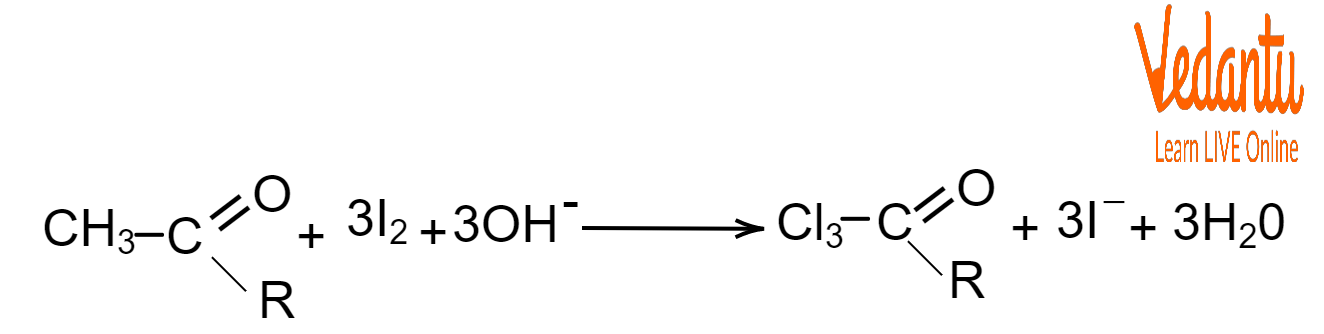
First Step of Iodoform Reaction
The second step is when the bond between CI3 and the rest of the molecule is broken. The hydroxide ion combines with the CI3 molecule to form a yellow precipitate called Triiodomethane (CHI3) or Iodoform with the salt of the acid.
$CI_3-CRO+HO^-\longrightarrow CHI_3+RCOO^-$
Iodoform Reaction with Alcohol
The triiodomethane (iodoform) reaction can be used to determine the presence of a CH3CH(OH) group in alcohols.
There are two seemingly different mixtures of reagents that can be used to carry out this reaction but are chemically equivalent.
This is the most chemically obvious method.
The iodine solution is added to a small amount of alcohol, followed by just enough sodium hydroxide solution to remove the colour from the iodine.
If nothing happens in cold conditions, it may be necessary to reheat the mixture gently.
Iodoform Reaction with Ethanol and Methanol
With ethanol, some secondary alcohols consisting of at least one methyl group in the alpha position are iodoform-positive. The Iodoform test is therefore useful in distinguishing ethanol from methanol, as ethanol is the only primary alcohol with a positive result.
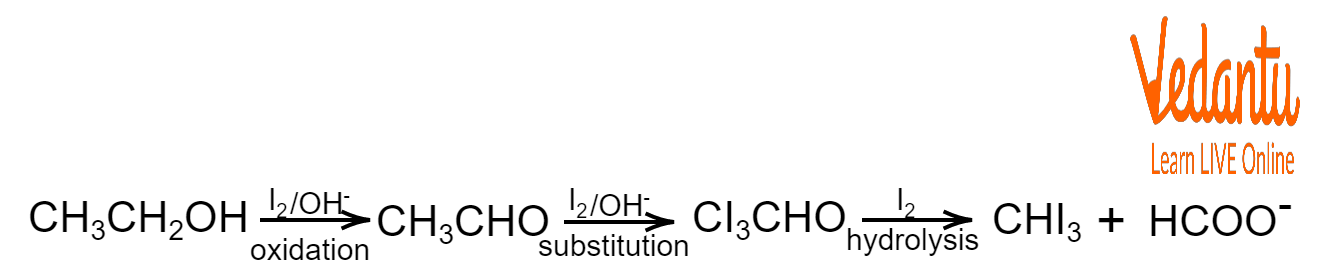
Reaction Equation for Test for Ethanol
When the ethanol undergoes the iodoform test, two further reaction steps are performed. In the preliminary stage, ethanol is oxidised to form CH3CHO and water.
$CH_3CH_2OH+HO^-\longrightarrow CH_3CHO+H_2O$$CH_3CHO\rightleftharpoons CH_2=CH-OH$
In the next step of the reaction, iodine replaces the hydrogen atoms in the alcohol compound. This process is repeated three times until all the hydrogen atoms associated with the carbon atom are replaced by iodine.
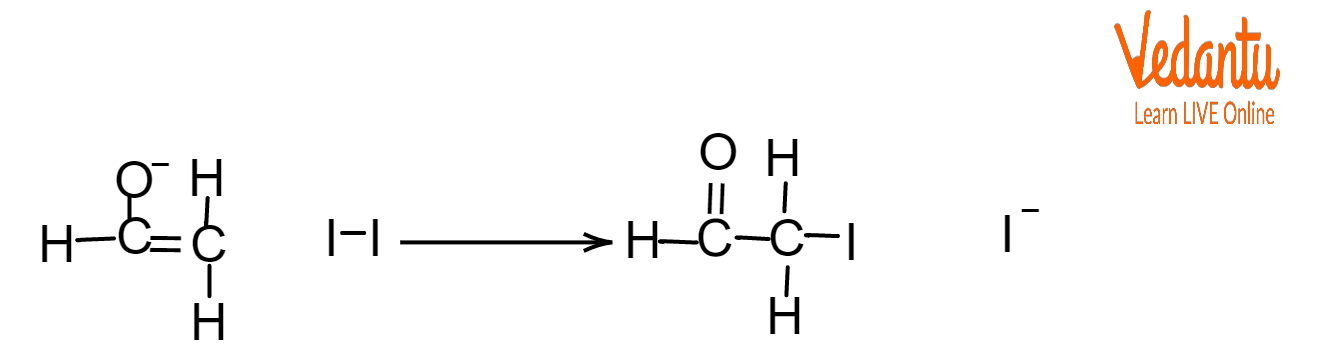
Second Step of Test for Ethanol
$CIH_2CHO+2I_2\rightarrow CI_3CHO+2I^-$
In the final step, the organic compound undergoes hydrolysis to form triiodomethane, a yellow precipitate, thus confirming the presence of ethanol.
$CI_3CHO+OH^-\longrightarrow CHI_3+CHOO^-$
No reaction occurs when methanol is treated with iodine and sodium hydroxide (NaOH).
Conclusion
When iodine and sodium hydroxide are added to the compound containing methyl ketone or secondary alcohol with a methyl group in the alpha position, a pale yellow precipitate of iodoform or triiodomethane is formed. It can be used to determine aldehydes or ketones. If an aldehyde is positive for iodoform, it must be acetaldehyde because it is the only aldehyde with a CH3C=O group.
FAQs on Iodoform Reactions - JEE Important Topic
1. What does the iodoform test indicate?
The iodoform test indicates that the presence of an aldehyde or ketone in which one of the groups is directly attached to the carbonyl carbon is the methyl group and such a ketone is called a methyl ketone. In the iodoform test, the unknown substance is allowed to react with a mixture of excess iodine and excess hydroxide.
2. Why does methanol not give a positive result in the iodoform test?
Methanol and propanol do not give a positive iodoform test because they do not have a methyl group attached to the carbon-containing OH group. 2-Propanol allows for the iodoform test because it contains a methyl group bonded to a carbon-containing OH group.


































