
Which of the following statement is not correct?
A.Phenol is neutralised by sodium carbonate.
B.Phenol is used to prepare analgesic drugs
C.Solubility of phenol in water is more than that of chlorobenzene
D.Boiling point of o-nitrophenol is lower than that of p-nitrophenol
Answer
232.8k+ views
Hint: We will check on every point if its correct or not. Hydroxy group of phenol has so many effects on its properties like solubility and boiling point. Phenol has many applications such as it can be used to make medicines. But it is a weaker acid as compared to carboxylic acids.
Complete Step-by-Step answer:
Phenols are weakly acidic in nature due to the presence of polar O-H group. They turn blue litmus paper red and react with alkali metals and alkaline metals to form their salts. So, we can say that phenol is a weaker acid than carboxylic acid, therefore it does not react with sodium carbonate and sodium bicarbonate and is not neutralised by sodium carbonate.
Analgesic reduce and abolish pain without causing impairment of consciousness, mental confusion or any disturbance in nervous system. Aspirin and paracetamol belong to the non-narcotic analgesics. These are phenolic compounds used to prepare such analgesic drugs. So, option (B) is correct.
Phenols form stronger hydrogen bonds just like alcohols. Thus, they are soluble in water and have higher boiling points. The ability of phenols to form strong hydrogen bonds also enhances their solubility in water. We can see that phenol dissolves to give a 9.3 percent solution in water, compared with a 3.6 percent solution for chlorobenzene in water. The association between water and phenol is unusually strong. When crystalline phenol is left in a humid environment, it picks up enough water from the air to form liquid droplets. So, option (C) is correct.
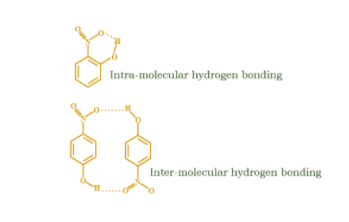
In ortho nitrophenol there is a possibility of intramolecular hydrogen bonding but for para nitrophenol there is possibility of intermolecular hydrogen bonding. So, o-nitrophenol due to intramolecular H-bonding acts as a monomer whereas for para isomer due to intermolecular H-bonding it can associate with other molecules and acts as like-polymer. Thus, the molecular weight of the system increases in case of para isomer and the boiling point also increases.So, option (D) is correct.
Hence, the correct option is (A).
Note: Hydrogen bonding is an electrostatic attraction between an electronegative element and Hydrogen atom attached to that electronegative element. Now if the hydrogen bonding occurs within a molecule, we call it as intramolecular hydrogen bonding but if it occurs within molecules, we call it as intermolecular hydrogen bonding.
Complete Step-by-Step answer:
Phenols are weakly acidic in nature due to the presence of polar O-H group. They turn blue litmus paper red and react with alkali metals and alkaline metals to form their salts. So, we can say that phenol is a weaker acid than carboxylic acid, therefore it does not react with sodium carbonate and sodium bicarbonate and is not neutralised by sodium carbonate.
Analgesic reduce and abolish pain without causing impairment of consciousness, mental confusion or any disturbance in nervous system. Aspirin and paracetamol belong to the non-narcotic analgesics. These are phenolic compounds used to prepare such analgesic drugs. So, option (B) is correct.
Phenols form stronger hydrogen bonds just like alcohols. Thus, they are soluble in water and have higher boiling points. The ability of phenols to form strong hydrogen bonds also enhances their solubility in water. We can see that phenol dissolves to give a 9.3 percent solution in water, compared with a 3.6 percent solution for chlorobenzene in water. The association between water and phenol is unusually strong. When crystalline phenol is left in a humid environment, it picks up enough water from the air to form liquid droplets. So, option (C) is correct.
In ortho nitrophenol there is a possibility of intramolecular hydrogen bonding but for para nitrophenol there is possibility of intermolecular hydrogen bonding. So, o-nitrophenol due to intramolecular H-bonding acts as a monomer whereas for para isomer due to intermolecular H-bonding it can associate with other molecules and acts as like-polymer. Thus, the molecular weight of the system increases in case of para isomer and the boiling point also increases.So, option (D) is correct.

Hence, the correct option is (A).
Note: Hydrogen bonding is an electrostatic attraction between an electronegative element and Hydrogen atom attached to that electronegative element. Now if the hydrogen bonding occurs within a molecule, we call it as intramolecular hydrogen bonding but if it occurs within molecules, we call it as intermolecular hydrogen bonding.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




