
Which of the following species are hypervalent?
1. \[Cl{O_4}^ - \] 2. \[B{F_3}\] 3. \[S{O_4}^{2 - }\] 4. \[C{O_3}^{2 - }\]
A. 1, 2, 3
B. 1, 3
C. 3, 4
D. 1, 2
Answer
233.1k+ views
Hint: A hypervalent molecule or a hypervalent species is a compound in which the given molecule contains one or more main group elements, and have more than eight electrons in their valence shell. This phenomenon is also commonly known as ‘expanded octet’.
Complete Step-by-Step answer:
To determine which of the given molecules are hypervalent, let us first understand the molecular structures of these compounds:
\[Cl{O_4}^ - \]
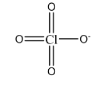
As we can observe in this structure, chlorine is forming 6 double bonds and 1 single bond. This brings the total number of electrons present in the valence shell of chlorine to be:
\[ = {\text{ }}6{\text{ }}\left( 4 \right){\text{ }} + {\text{ }}1{\text{ }}\left( 2 \right){\text{ }} = {\text{ }}12\] electrons
Hence, the given species is hypervalent
\[B{F_3}\]
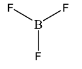
As we can observe in this structure, boron is forming 3 single bonds. This brings the total number of atoms present in the valence shell of boron to be:
\[ = {\text{ }}3\left( 2 \right){\text{ }} = {\text{ }}6\] electrons
Hence, the given species is not hypervalent
\[Cl{O_4}^ - \]
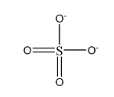
As we can observe in this structure, sulphur is forming 2 double bonds and 2 single bonds. This brings the total number of electrons present in the valence shell of sulphur to be:
\[ = {\text{ }}2{\text{ }}\left( 4 \right){\text{ }} + {\text{ }}2{\text{ }}\left( 2 \right){\text{ }} = {\text{ }}10\] electrons
Hence, the given species is hypervalent
\[C{O_3}^{2 - }\]
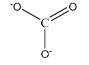
As we can observe in this structure, carbon is forming 2 single bonds and 1 double bond. This brings the total number of atoms present in the valence shell of carbon to be:
\[ = {\text{ }}2\left( 2 \right){\text{ }} + {\text{ }}1\left( 4 \right){\text{ }} = {\text{ }}8\] electrons
Hence, the given species is not hypervalent
Hence, we can conclude that, the species that are hypervalent are \[Cl{O_4}^ - \] and \[Cl{O_4}^ - \]
Hence, Option B is the correct option.
Note: Early considerations of the geometry of hypervalent molecules returned familiar arrangements that were well explained by the VSEPR model for atomic bonding. In order to account for the observed bond angles, bond lengths and apparent violation of the Lewis octet rule, several alternative models have been proposed.
Complete Step-by-Step answer:
To determine which of the given molecules are hypervalent, let us first understand the molecular structures of these compounds:
\[Cl{O_4}^ - \]

As we can observe in this structure, chlorine is forming 6 double bonds and 1 single bond. This brings the total number of electrons present in the valence shell of chlorine to be:
\[ = {\text{ }}6{\text{ }}\left( 4 \right){\text{ }} + {\text{ }}1{\text{ }}\left( 2 \right){\text{ }} = {\text{ }}12\] electrons
Hence, the given species is hypervalent
\[B{F_3}\]

As we can observe in this structure, boron is forming 3 single bonds. This brings the total number of atoms present in the valence shell of boron to be:
\[ = {\text{ }}3\left( 2 \right){\text{ }} = {\text{ }}6\] electrons
Hence, the given species is not hypervalent
\[Cl{O_4}^ - \]

As we can observe in this structure, sulphur is forming 2 double bonds and 2 single bonds. This brings the total number of electrons present in the valence shell of sulphur to be:
\[ = {\text{ }}2{\text{ }}\left( 4 \right){\text{ }} + {\text{ }}2{\text{ }}\left( 2 \right){\text{ }} = {\text{ }}10\] electrons
Hence, the given species is hypervalent
\[C{O_3}^{2 - }\]

As we can observe in this structure, carbon is forming 2 single bonds and 1 double bond. This brings the total number of atoms present in the valence shell of carbon to be:
\[ = {\text{ }}2\left( 2 \right){\text{ }} + {\text{ }}1\left( 4 \right){\text{ }} = {\text{ }}8\] electrons
Hence, the given species is not hypervalent
Hence, we can conclude that, the species that are hypervalent are \[Cl{O_4}^ - \] and \[Cl{O_4}^ - \]
Hence, Option B is the correct option.
Note: Early considerations of the geometry of hypervalent molecules returned familiar arrangements that were well explained by the VSEPR model for atomic bonding. In order to account for the observed bond angles, bond lengths and apparent violation of the Lewis octet rule, several alternative models have been proposed.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




