
Which of the following is not the usual method for preparation of primary amine?
A. Hofmann’s method
B. Curtius reaction
C. Schmidt reaction
D. Friedel-craft reaction
Answer
233.1k+ views
Hint: Primary amine can be formed when one hydrogen atom of ammonia is replaced by alkyl group or any aryl group such as RNH2 where R can be an aromatic group or any alkyl group.
Complete Step by Step Solution:
Primary amine (1°) can be prepared by Hofmann reaction when primary amide react with aqueous solution of sodium hydroxide or potassium hydroxide and bromide and thus, give suitable primary amine such as
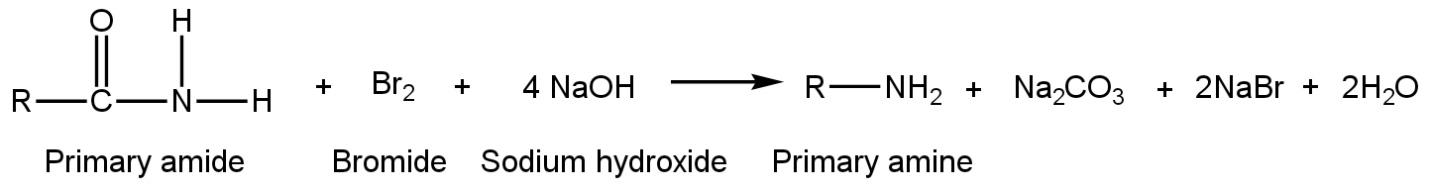
Curtius reaction or Curtius degradation is also helpful to prepare primary amine. To prepare primary amine from this reaction, acyl azide undergoes thermal decomposition to give isocyanate after losing N2. Then isocyanate is made to react with nucleophile H2O to give primary amine such as
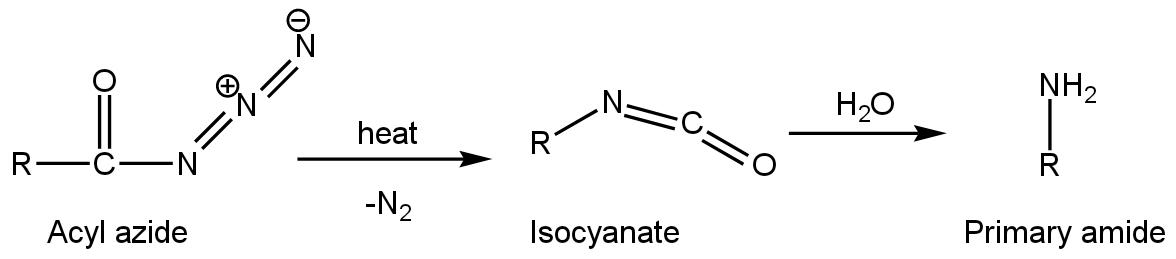
Primary amine can also be prepared from Schmidt reactions. This is quite a simple and sorted method. In this reaction carboxylic acid derivatives react with azide group in the presence of acidic condition (H+ ion) to give suitable primary amine with the release of nitrogen and Carbon dioxide such as
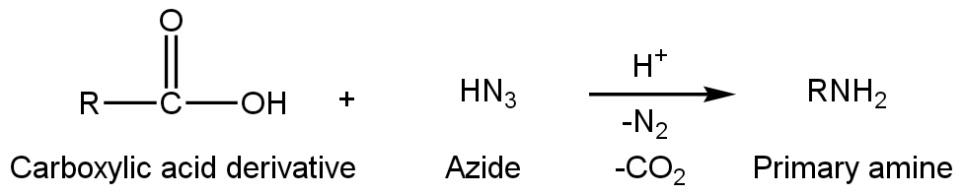
Whereas Friedel-crafts reaction is not a suitable method to prepare primary amine. Friedel-crafts reaction is only used for preparation of alkyl benzene or acetophenone.
Thus, the correct answer is D, as Friedel-crafts reaction is not the usual method for preparation of primary amine.
Note: Friedel-crafts reaction is only used to attached substituents on aromatic rings. There are two types of Friedel-crafts reaction one is alkylation and acylation reaction. In alkylation reaction alkyl groups are substitutes and acylation reaction acyl group substitutes.
Complete Step by Step Solution:
Primary amine (1°) can be prepared by Hofmann reaction when primary amide react with aqueous solution of sodium hydroxide or potassium hydroxide and bromide and thus, give suitable primary amine such as

Curtius reaction or Curtius degradation is also helpful to prepare primary amine. To prepare primary amine from this reaction, acyl azide undergoes thermal decomposition to give isocyanate after losing N2. Then isocyanate is made to react with nucleophile H2O to give primary amine such as

Primary amine can also be prepared from Schmidt reactions. This is quite a simple and sorted method. In this reaction carboxylic acid derivatives react with azide group in the presence of acidic condition (H+ ion) to give suitable primary amine with the release of nitrogen and Carbon dioxide such as

Whereas Friedel-crafts reaction is not a suitable method to prepare primary amine. Friedel-crafts reaction is only used for preparation of alkyl benzene or acetophenone.
Thus, the correct answer is D, as Friedel-crafts reaction is not the usual method for preparation of primary amine.
Note: Friedel-crafts reaction is only used to attached substituents on aromatic rings. There are two types of Friedel-crafts reaction one is alkylation and acylation reaction. In alkylation reaction alkyl groups are substitutes and acylation reaction acyl group substitutes.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




