
Which of the following is known as invert soap?
A. Pentaerythritol monostearate
B. Sodium stearyl sulphate
C. Trimethyl stearyl ammonium bromide
D. Ethoxylated nonylphenol
Answer
233.1k+ views
Hint: Active part of the invert soap molecule is different than the normal soap molecule. Invert soap is cationic detergent.
Complete step by step answer:
Positively charged ions are known as cations while negatively charged ions are known as anions.
Soap containing anionic surface molecule as the active part is known as normal soap while invert soap has cationic surface molecule as the active part.
The molecular formula of Pentaerythritol Monostearate is ${{\text{C}}_{{\text{23}}}}{{\text{H}}_{{\text{46}}}}{{\text{O}}_{\text{5}}}$. It is not classified as invert soap as it does not have a cationic surface molecule, as the active part.

So, option (A) Pentaerythritol monostearate is not the correct answer.
The molecular formula of Sodium stearyl sulphate is ${{\text{C}}_{{\text{18}}}}{{\text{H}}_{{\text{37}}}}{\text{Na}}{{\text{O}}_{\text{4}}}{\text{S}}$ . This is alkyl sulphates detergent. Alkyl sulphate detergent is the anionic type of detergent where stearyl sulphate is an active anionic part on the surface of the molecule. It is a normal soap.
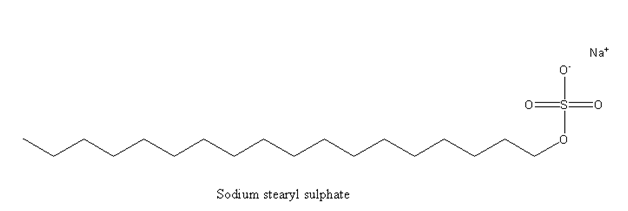
So, option (B) Sodium stearyl sulphate is not the correct answer.
The molecular formula of Trimethyl stearyl ammonium bromide is ${{\text{C}}_{{\text{21}}}}{{\text{H}}_{{\text{46}}}}{\text{NBr}}$.The ammonium part of this soap is positively charged ion. As It has an active cationic part on the surface of the molecule it is known as invert soap.
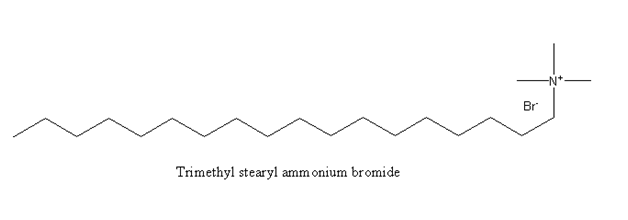
Hence, option (C) Trimethyl stearyl ammonium bromide is the correct option.
The molecular formula of Ethoxylated nonylphenol detergent is ${{\text{C}}_{19}}{{\text{H}}_{{\text{32}}}}{{\text{O}}_3}$. It is a type of non-ionic detergent
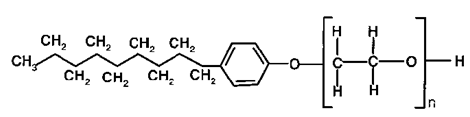
So, option (D) Ethoxylated nonylphenol is not the correct answer.
Thus, the correct option is C.
Note: Detergents act as cleansing agents. Depending on the active part on the surface of molecules, detergents are classified as cationic detergents, anionic detergents and non-ionic detergents.
Complete step by step answer:
Positively charged ions are known as cations while negatively charged ions are known as anions.
Soap containing anionic surface molecule as the active part is known as normal soap while invert soap has cationic surface molecule as the active part.
The molecular formula of Pentaerythritol Monostearate is ${{\text{C}}_{{\text{23}}}}{{\text{H}}_{{\text{46}}}}{{\text{O}}_{\text{5}}}$. It is not classified as invert soap as it does not have a cationic surface molecule, as the active part.

So, option (A) Pentaerythritol monostearate is not the correct answer.
The molecular formula of Sodium stearyl sulphate is ${{\text{C}}_{{\text{18}}}}{{\text{H}}_{{\text{37}}}}{\text{Na}}{{\text{O}}_{\text{4}}}{\text{S}}$ . This is alkyl sulphates detergent. Alkyl sulphate detergent is the anionic type of detergent where stearyl sulphate is an active anionic part on the surface of the molecule. It is a normal soap.

So, option (B) Sodium stearyl sulphate is not the correct answer.
The molecular formula of Trimethyl stearyl ammonium bromide is ${{\text{C}}_{{\text{21}}}}{{\text{H}}_{{\text{46}}}}{\text{NBr}}$.The ammonium part of this soap is positively charged ion. As It has an active cationic part on the surface of the molecule it is known as invert soap.

Hence, option (C) Trimethyl stearyl ammonium bromide is the correct option.
The molecular formula of Ethoxylated nonylphenol detergent is ${{\text{C}}_{19}}{{\text{H}}_{{\text{32}}}}{{\text{O}}_3}$. It is a type of non-ionic detergent

So, option (D) Ethoxylated nonylphenol is not the correct answer.
Thus, the correct option is C.
Note: Detergents act as cleansing agents. Depending on the active part on the surface of molecules, detergents are classified as cationic detergents, anionic detergents and non-ionic detergents.
Recently Updated Pages
JEE Main 2026 Session 2 Registration Open, Exam Dates, Syllabus & Eligibility

JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

Trending doubts
JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

In Carius method of estimation of halogens 015g of class 11 chemistry JEE_Main

Understanding Average and RMS Value in Electrical Circuits

Understanding Collisions: Types and Examples for Students

Ideal and Non-Ideal Solutions Explained for Class 12 Chemistry

Understanding Atomic Structure for Beginners

Other Pages
JEE Advanced Weightage 2025 Chapter-Wise for Physics, Maths and Chemistry

NCERT Solutions For Class 11 Chemistry in Hindi Chapter 1 Some Basic Concepts of Chemistry (2025-26)

NCERT Solutions For Class 11 Chemistry in Hindi Chapter 8 Redox Reactions (2025-26)

An ideal gas is at pressure P and temperature T in class 11 chemistry JEE_Main

Inductive Effect and Its Role in Acidic Strength

Degree of Dissociation: Meaning, Formula, Calculation & Uses




