
Which of the following electromagnetic radiations have the smallest wavelength?
A) Ultraviolet waves
B) X-rays
C) Gamma rays
D) Microwaves
Answer
233.1k+ views
Hint: We solve this question by recalling the light spectrum. The rays with higher frequency have a lower wavelength and rays with higher wavelength have a lower frequency. Frequency and wavelength are inversely proportional to each other. Refer to the diagram in a detailed solution for a better understanding.
Complete step by step solution:
In the electromagnetic spectrum there are visible and non-visible rays. The visible light region is in the middle of the spectrum. All the radioactive rays are situated on the extreme right of the spectrum and microwaves are located on the left of the spectrum.
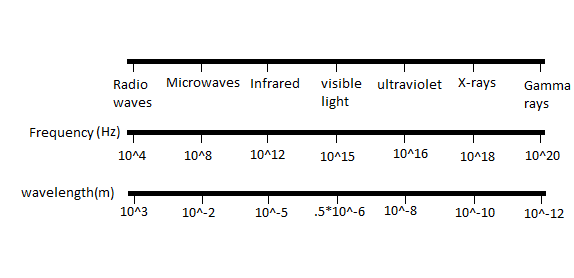
The trend followed by the electromagnetic spectrum is that as we move from the left to right on an electromagnetic spectrum the frequency increases and the wavelength of the rays increase. So, the rays on the extreme right have the lowest wavelength.
All the radioactive rays are present on the extreme right of an electromagnetic spectrum out of which Gamma rays are the last then X-rays and then ultraviolet rays.
Hence Gamma rays have the lowest or smallest wavelength.
Option (C), Gamma rays is the correct answer.
Additional information: The increasing order of frequency of the rays in the electromagnetic spectrum is as follows Radio waves, Microwaves, Infrared, visible light, ultraviolet waves, X-rays, Gamma rays. The reverse order of this will give us the increasing order of wavelength. Hence frequency and wavelength are inversely proportional to each other.
Note: Students might get confused with the trend on the electromagnetic spectrum by taking wavelength as frequency and frequency and wavelength. It is to be remembered clearly that the wavelength decreases as we move from the left to right on an electromagnetic spectrum.
Complete step by step solution:
In the electromagnetic spectrum there are visible and non-visible rays. The visible light region is in the middle of the spectrum. All the radioactive rays are situated on the extreme right of the spectrum and microwaves are located on the left of the spectrum.

The trend followed by the electromagnetic spectrum is that as we move from the left to right on an electromagnetic spectrum the frequency increases and the wavelength of the rays increase. So, the rays on the extreme right have the lowest wavelength.
All the radioactive rays are present on the extreme right of an electromagnetic spectrum out of which Gamma rays are the last then X-rays and then ultraviolet rays.
Hence Gamma rays have the lowest or smallest wavelength.
Option (C), Gamma rays is the correct answer.
Additional information: The increasing order of frequency of the rays in the electromagnetic spectrum is as follows Radio waves, Microwaves, Infrared, visible light, ultraviolet waves, X-rays, Gamma rays. The reverse order of this will give us the increasing order of wavelength. Hence frequency and wavelength are inversely proportional to each other.
Note: Students might get confused with the trend on the electromagnetic spectrum by taking wavelength as frequency and frequency and wavelength. It is to be remembered clearly that the wavelength decreases as we move from the left to right on an electromagnetic spectrum.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Dual Nature of Radiation and Matter Class 12 Physics Chapter 11 CBSE Notes - 2025-26

Understanding Uniform Acceleration in Physics

Understanding the Electric Field of a Uniformly Charged Ring

JEE Advanced Weightage 2025 Chapter-Wise for Physics, Maths and Chemistry

Derivation of Equation of Trajectory Explained for Students




