
Which of the following configurations show the second excitation state of iodine?
A. 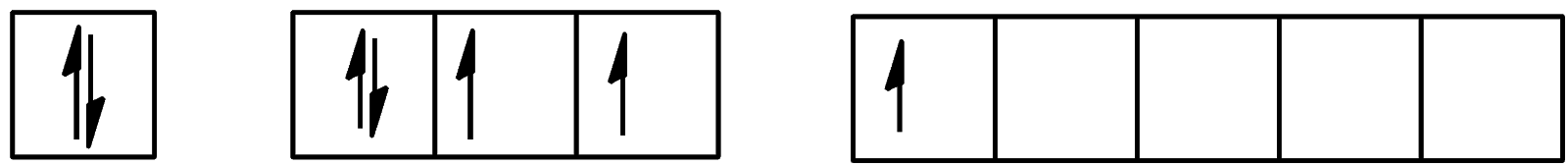
B. 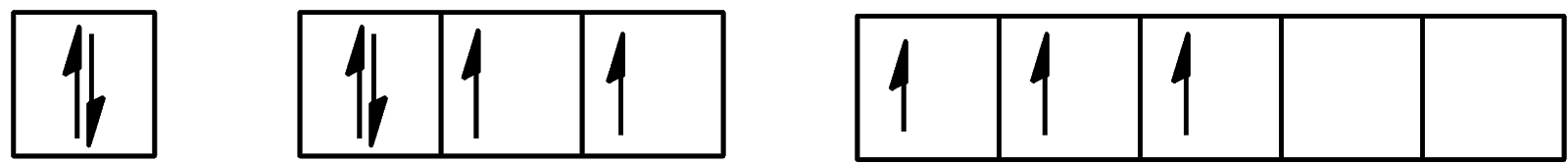
C. 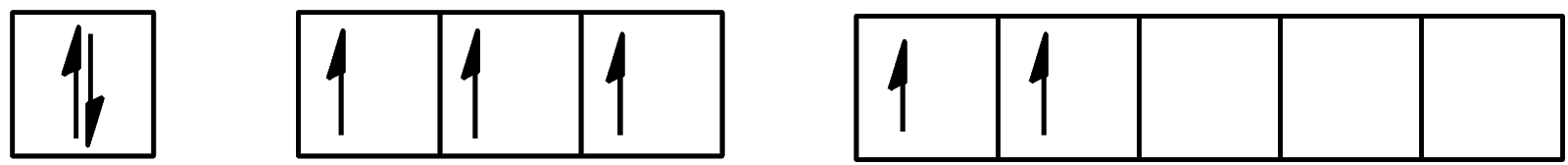
D. 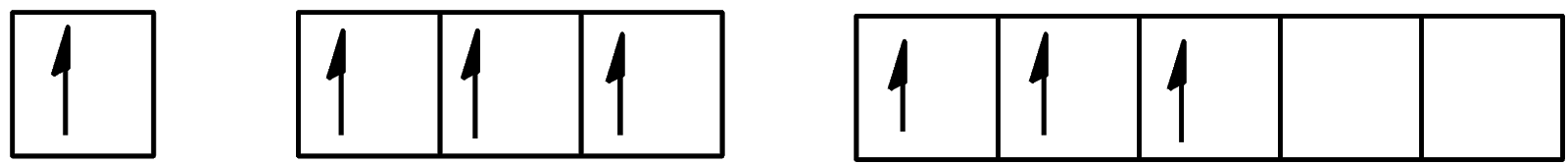
Answer
233.1k+ views
Hint: Think about the atomic number of iodine and the number of electrons that are present in its outermost shell. Determine the second excitation state according to this.
Complete step by step solution:
We know that the atomic number of iodine is 53, and its electronic configuration is denoted by $[Kr]4{{d}^{10}}5{{s}^{2}}5{{p}^{5}}$. We will only consider the outermost valence orbitals of the atom, they are $5{{s}^{2}}5{{p}^{5}}$.
The atom is considered to enter its first excitation state when one electron from the outermost shell occupies a previously unoccupied orbital with the lowest energy. Here, the unoccupied orbital belongs to the $5d$ orbital. So, the electron from $5p$ will move to the $5d$ orbital. The same will happen in the second excitation state. An electron from $5p$ will move to the $5d$ orbital during this process too. Now, let us look at the ground state, the first excitation state, and the second excitation state.
- For the ground state, the electronic configuration will be:

We see that no electrons are present in the d-orbital.
- For the first excitation state, the configuration will be:
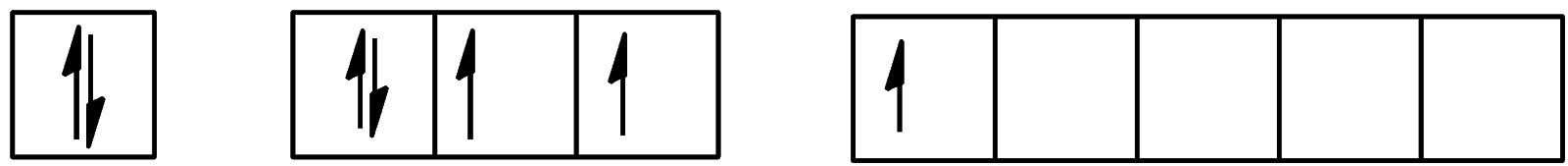
We can see that one electron is present in the d-orbital and 4 electrons are present in the p-orbital. The configuration will be $[Kr]4{{d}^{10}}5{{s}^{2}}5{{p}^{4}}5{{d}^{1}}$.
- For the second excitation state, the configuration will be:
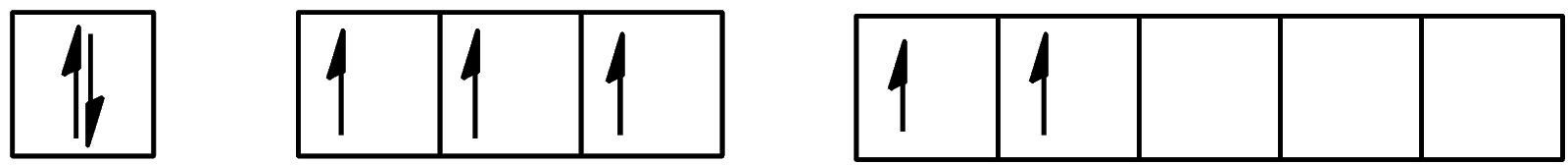
We can see that 2 electrons are present in the d-orbital and 3 electrons are present in the p-orbital. The configuration will be $[Kr]4{{d}^{10}}5{{s}^{2}}5{{p}^{3}}5{{d}^{2}}$.
Hence, the correct answer to this question is option C.
Note: Remember that the electron that moves to the higher orbital will be the electron that has been written at the end while drawing the electronic configuration using Hund’s rule and Pauli’s exclusion principle. So the unpaired electron from the $5{{p}_{z}}$ orbital will not move to the higher orbital but the paired electron from the $5{{p}_{y}}$ will move first and then the paired electron from the $5{{p}_{x}}$ orbital.
Complete step by step solution:
We know that the atomic number of iodine is 53, and its electronic configuration is denoted by $[Kr]4{{d}^{10}}5{{s}^{2}}5{{p}^{5}}$. We will only consider the outermost valence orbitals of the atom, they are $5{{s}^{2}}5{{p}^{5}}$.
The atom is considered to enter its first excitation state when one electron from the outermost shell occupies a previously unoccupied orbital with the lowest energy. Here, the unoccupied orbital belongs to the $5d$ orbital. So, the electron from $5p$ will move to the $5d$ orbital. The same will happen in the second excitation state. An electron from $5p$ will move to the $5d$ orbital during this process too. Now, let us look at the ground state, the first excitation state, and the second excitation state.
- For the ground state, the electronic configuration will be:

We see that no electrons are present in the d-orbital.
- For the first excitation state, the configuration will be:

We can see that one electron is present in the d-orbital and 4 electrons are present in the p-orbital. The configuration will be $[Kr]4{{d}^{10}}5{{s}^{2}}5{{p}^{4}}5{{d}^{1}}$.
- For the second excitation state, the configuration will be:

We can see that 2 electrons are present in the d-orbital and 3 electrons are present in the p-orbital. The configuration will be $[Kr]4{{d}^{10}}5{{s}^{2}}5{{p}^{3}}5{{d}^{2}}$.
Hence, the correct answer to this question is option C.
Note: Remember that the electron that moves to the higher orbital will be the electron that has been written at the end while drawing the electronic configuration using Hund’s rule and Pauli’s exclusion principle. So the unpaired electron from the $5{{p}_{z}}$ orbital will not move to the higher orbital but the paired electron from the $5{{p}_{y}}$ will move first and then the paired electron from the $5{{p}_{x}}$ orbital.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




