
Which of the following compounds has zero dipole moment?
(A) ${ 1,4-dichlorobenzene }$
(B) ${ 1,2-dichlorobenzene }$
(C) ${ 1,3-dichlorobenzene }$
(D) ${ 1-chloro-2-methylbenzene }$
Answer
521k+ views
Hint: The bond dipole moment uses the idea of an electric dipole moment to measure the polarity of a chemical bond within a molecule. It occurs whenever there is a separation of positive and negative charges. The bond dipole μ is given by: ${ \mu =\delta d }$
Complete step by step solution:
As there are two Cl groups present in ortho makes an acute angle according to the equation;
Dipole moment, D = $ \sqrt { { d }_{ 1 }^{ 2 }{ +d }_{ 2 }^{ 2 }{ +d }_{ 1 }{ d }_{ 2 }{ cosx } }$
where, x = angle between the groups
A.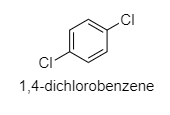
Here, the dipole moment is ${ 0 }$ as ${ Cos90 }^{ \circ }$ = ${ 0 }$.
B.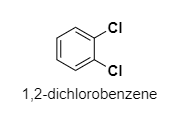
The dipole moment of ${ 1,2-dichlorobenzene }$ is ${ 2.54D }$,so
C.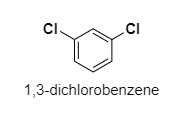
The dipole moment of ${ 1,3-dichlorobenzene }$ is ${ 1.72D }$.
D.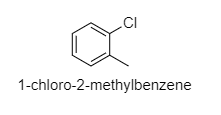
Here, there will be some dipole moment, not zero.
The order of dipole moment is: ${ 1,2-dichlorobenzene }$ ${ 1,3-dichlorobenzene }$ ${ 1,4-dichlorobenzene }$.
Trans-dichlorobenzene has zero dipole moment as it is symmetrical and the dipole moment will cancel out.
Hence, the correct option is A.
> Factors affecting dipole moment:
1) Polarity of molecule
2) Magnitude of charge
3) Geometry of molecule
4) The boiling point of the element.
5) Position of bond and lone pair of electrons in a molecule.
6) The dipole moment depends upon the bond length.
Note: The possibility to make a mistake is that you may choose option C. But in ${ 1,3-dichlorobenzene }$ they are not parallel and hence they will not cancel out completely, it has some dipole moment.
Complete step by step solution:
As there are two Cl groups present in ortho makes an acute angle according to the equation;
Dipole moment, D = $ \sqrt { { d }_{ 1 }^{ 2 }{ +d }_{ 2 }^{ 2 }{ +d }_{ 1 }{ d }_{ 2 }{ cosx } }$
where, x = angle between the groups
A.

Here, the dipole moment is ${ 0 }$ as ${ Cos90 }^{ \circ }$ = ${ 0 }$.
B.

The dipole moment of ${ 1,2-dichlorobenzene }$ is ${ 2.54D }$,so
C.

The dipole moment of ${ 1,3-dichlorobenzene }$ is ${ 1.72D }$.
D.

Here, there will be some dipole moment, not zero.
The order of dipole moment is: ${ 1,2-dichlorobenzene }$ ${ 1,3-dichlorobenzene }$ ${ 1,4-dichlorobenzene }$.
Trans-dichlorobenzene has zero dipole moment as it is symmetrical and the dipole moment will cancel out.
Hence, the correct option is A.
> Factors affecting dipole moment:
1) Polarity of molecule
2) Magnitude of charge
3) Geometry of molecule
4) The boiling point of the element.
5) Position of bond and lone pair of electrons in a molecule.
6) The dipole moment depends upon the bond length.
Note: The possibility to make a mistake is that you may choose option C. But in ${ 1,3-dichlorobenzene }$ they are not parallel and hence they will not cancel out completely, it has some dipole moment.
Recently Updated Pages
JEE Main 2026 Session 2 Registration Open, Exam Dates, Syllabus & Eligibility

JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

Trending doubts
Understanding Average and RMS Value in Electrical Circuits

Ideal and Non-Ideal Solutions Explained for Class 12 Chemistry

Understanding Atomic Structure for Beginners

Understanding Elastic Collisions in Two Dimensions

For pure water A pH increases while pOH decreases with class 11 chemistry JEE_Main

Which of the following is most stable A Sn2+ B Ge2+ class 11 chemistry JEE_Main

Other Pages
NCERT Solutions For Class 11 Chemistry in Hindi Chapter 8 Redox Reactions (2025-26)

An ideal gas is at pressure P and temperature T in class 11 chemistry JEE_Main

In Carius method of estimation of halogens 015g of class 11 chemistry JEE_Main

Understanding Collisions: Types and Examples for Students

NCERT Solutions For Class 11 Chemistry in Hindi Chapter 1 Some Basic Concepts of Chemistry (2025-26)

Happy New Year Wishes 2026 – 100+ Messages, Quotes, Shayari, Images & Status in All Languages




