
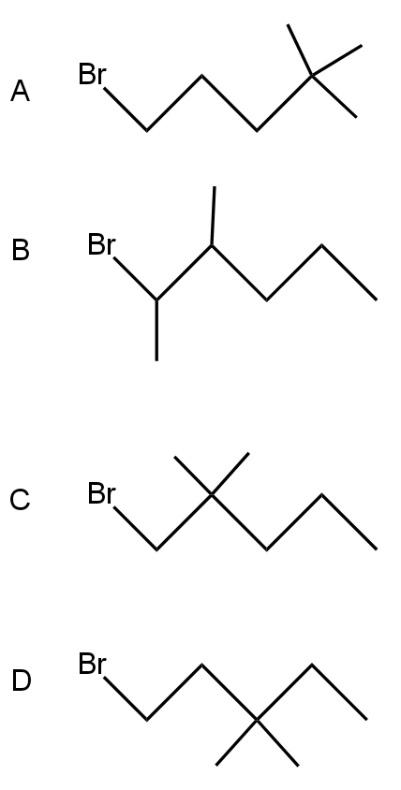
Which compound undergoes nucleophilic substitution with \[NaCN\]at the faster rate?
Answer
232.8k+ views
Hint: In the given options, bromine is bonded which is a good leaving group. Thus, it leaves compounds easily and generates carbonation. This carbonation will get attacked by nucleophiles. And the rate of this nucleophile attack will be greater at the carbonation which is surrounded with less bulky groups (less steric hindrance).
Complete Step by Step Solution:
Reagent \[NaCN\] is a strong nucleophile. In a nucleophilic substitution reaction, this nucleophile substitutes the nucleophile present in the given compound. Bromine's whose electronegativity is quite high as compared to its substrate (C) is a good leaving group and tends to attain a negative charge after attracting bond electrons towards itself with an inductive effect. This is the reason bromine is a good nucleophile.
As bromine (nucleophile) is a good leaving group thus from all the compounds given in the option, bromine will leave taking bond electron with itself and generating carbonation such as
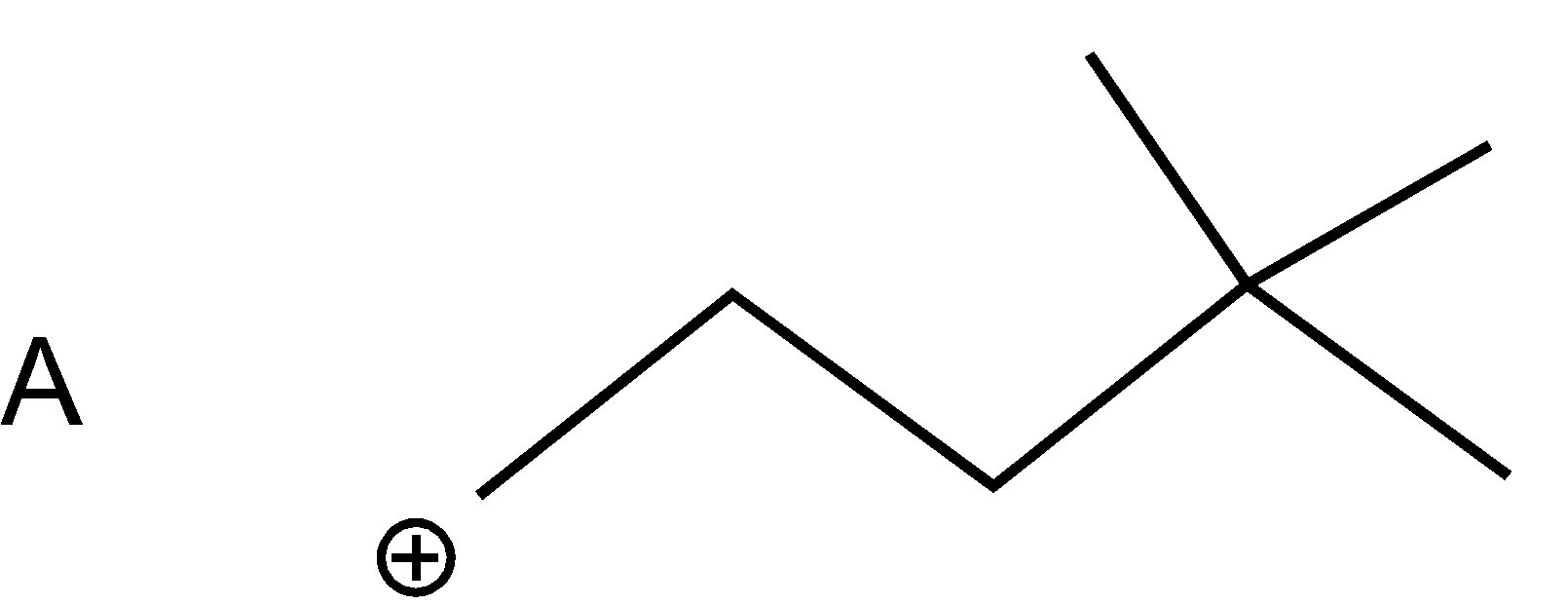
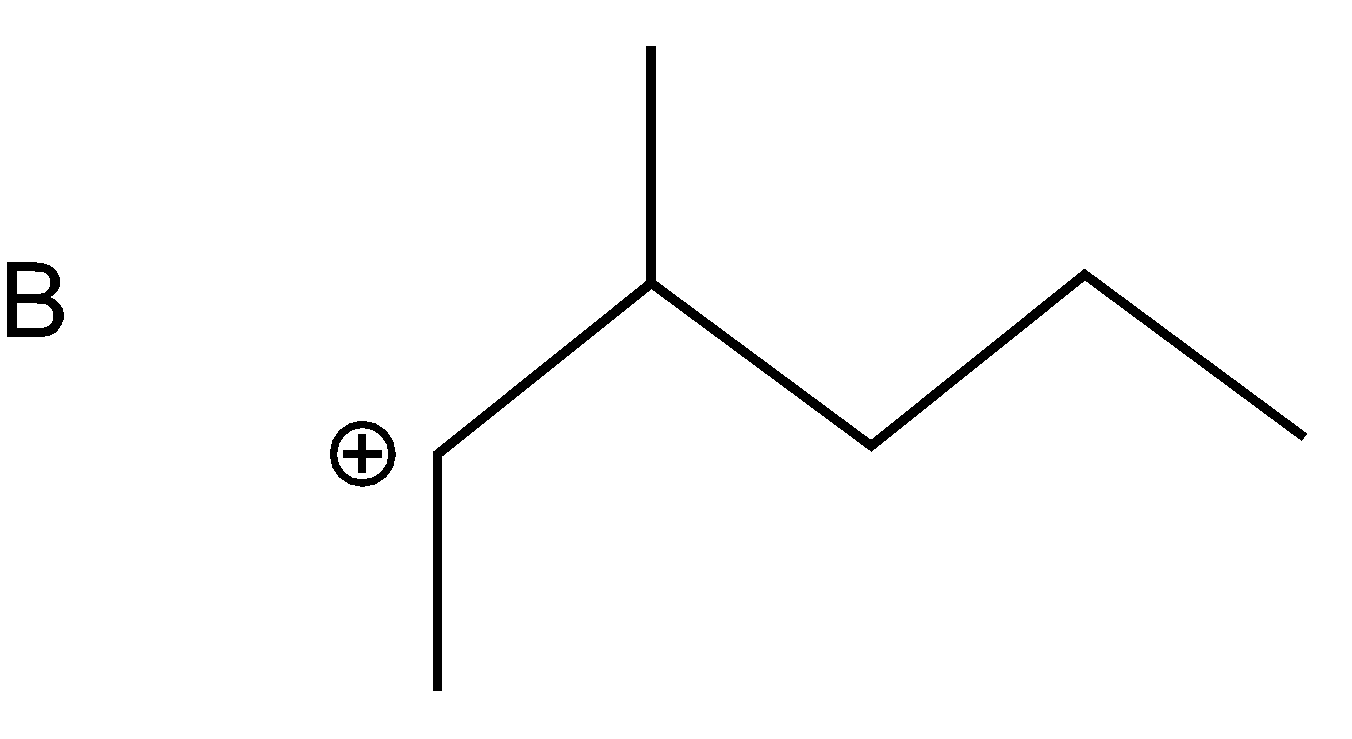
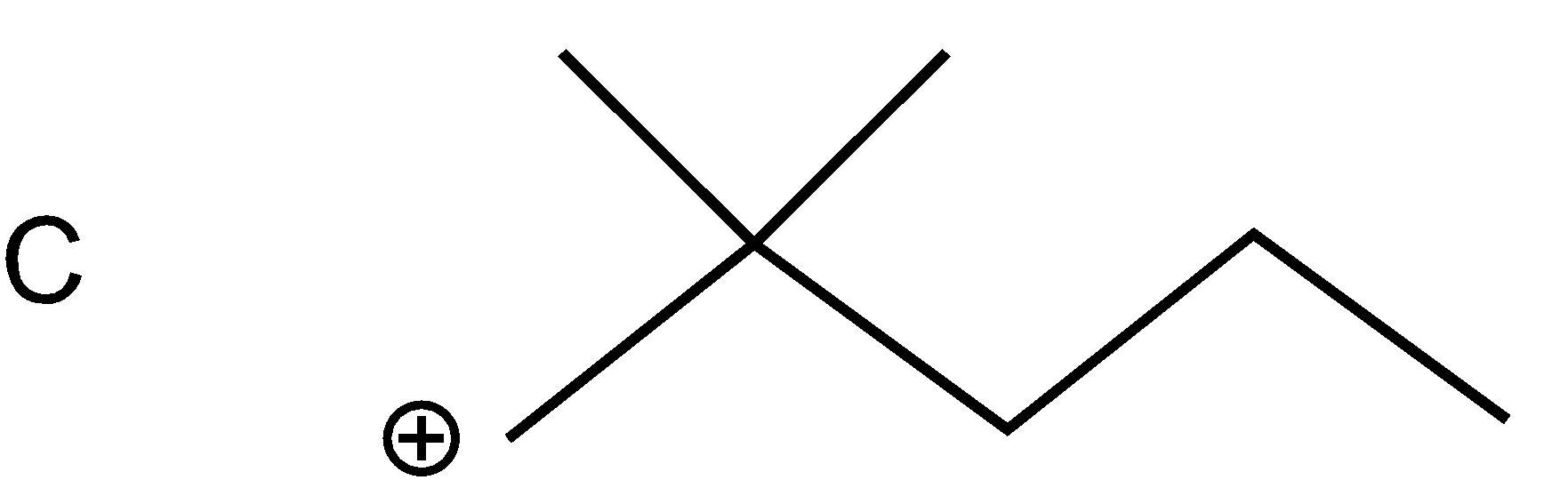
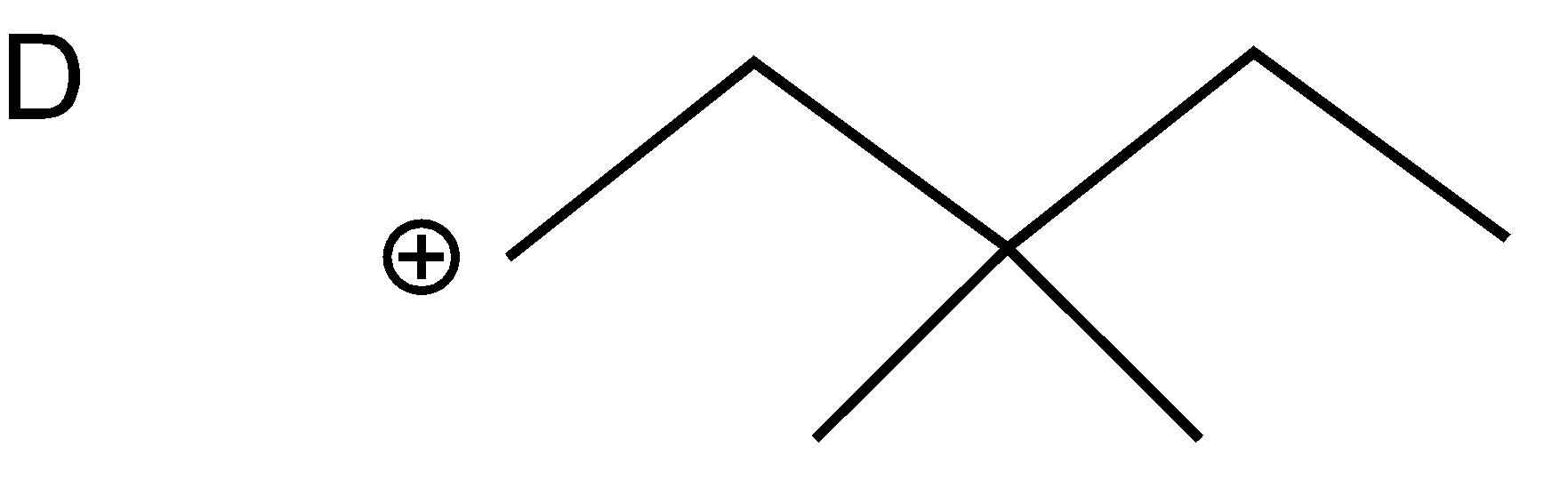
As a nucleophile leaves the compound, carbonation will form at which another nucleophile \[(C{{N}^{-}})\]will attack. Now nucleophiles attack fastly on that cation which is surrounded with a smaller number of groups (at the site of less steric hindrance) and show nucleophilic substitution reaction.
Compound A cation is less hindered thus, nucleophile firstly prefers to attack that compound. Further nucleophile attack on B compound as the bulky groups are far way the action. Compounds D and C are the compounds at which nucleophiles attack with difficulty.
Thus, the correct option is A.
Note: It is important to note that when a compound substrate is bonded with a strong base. Then this base tends to protonate through the hydrogen of carbon adjacent to the substrate and a new one more bond between substrate carbon and carbon to which protons get abstracted (alkane changes to alkene). This is an elimination reaction. If the substrate is bonded to the leaving group (strong nucleophile) thus a nucleophile takes the place of the leaving group and this is a substitution reaction.
Complete Step by Step Solution:
Reagent \[NaCN\] is a strong nucleophile. In a nucleophilic substitution reaction, this nucleophile substitutes the nucleophile present in the given compound. Bromine's whose electronegativity is quite high as compared to its substrate (C) is a good leaving group and tends to attain a negative charge after attracting bond electrons towards itself with an inductive effect. This is the reason bromine is a good nucleophile.
As bromine (nucleophile) is a good leaving group thus from all the compounds given in the option, bromine will leave taking bond electron with itself and generating carbonation such as




As a nucleophile leaves the compound, carbonation will form at which another nucleophile \[(C{{N}^{-}})\]will attack. Now nucleophiles attack fastly on that cation which is surrounded with a smaller number of groups (at the site of less steric hindrance) and show nucleophilic substitution reaction.
Compound A cation is less hindered thus, nucleophile firstly prefers to attack that compound. Further nucleophile attack on B compound as the bulky groups are far way the action. Compounds D and C are the compounds at which nucleophiles attack with difficulty.
Thus, the correct option is A.
Note: It is important to note that when a compound substrate is bonded with a strong base. Then this base tends to protonate through the hydrogen of carbon adjacent to the substrate and a new one more bond between substrate carbon and carbon to which protons get abstracted (alkane changes to alkene). This is an elimination reaction. If the substrate is bonded to the leaving group (strong nucleophile) thus a nucleophile takes the place of the leaving group and this is a substitution reaction.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




