
Which amino acids have an aromatic ring?
A. Alanine
B. Glycine
C. Tyrosine
D. Lysine
Answer
232.8k+ views
Hint: An aromatic substance is referred to as having an aromatic ring. The molecules that make up proteins have both an acid and an ammoniated group. A peptide bond is created when the acid and ammoniated group lose water. The amino acids are found in zwitter ionic form, where each molecule has a distinct charge.
Complete Step by Step Solution:
An aromatic ring makes up aromatic amino acids. Among the 20 present, there are 3 aromatic amino acids: phenylaniline, tyrosine, and tryptophan. All of the amino acids are necessary. We must obtain essential amino acids through dietary supplements since our bodies cannot generate them. Different illnesses are brought on by a deficiency of necessary amino acids. The absence of phenylaniline hydrolysis, which results in phenylketonuria, is typically the cause of phenylaniline insufficiency. Stunted growth results from tyrosine insufficiency, and Blue diaper syndrome from tryptophan shortage. Tyrosine has a 4-hydroxyphenylalanine structure, therefore an aniline group has a phenyl ring that is aromatic by nature. Foods high in protein are where tyrosine is found. The structure of Tyrosine is given as:
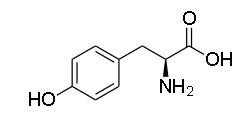
Hence, the correct option is C. Tyrosine.
Additional Information: The structure of the other three compounds are as follows:
The structure of Lysine:
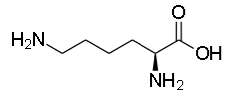
The structure of Glycine:
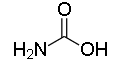
The structure of Alanine:
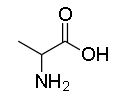
Note: The thing to note here is that there are only three aromatic amino acids among the 20 amino acids. The remaining three are Glycine, Alanine and Lysine and they all form a straight chain and no aromatic rings are there in their structure.
Complete Step by Step Solution:
An aromatic ring makes up aromatic amino acids. Among the 20 present, there are 3 aromatic amino acids: phenylaniline, tyrosine, and tryptophan. All of the amino acids are necessary. We must obtain essential amino acids through dietary supplements since our bodies cannot generate them. Different illnesses are brought on by a deficiency of necessary amino acids. The absence of phenylaniline hydrolysis, which results in phenylketonuria, is typically the cause of phenylaniline insufficiency. Stunted growth results from tyrosine insufficiency, and Blue diaper syndrome from tryptophan shortage. Tyrosine has a 4-hydroxyphenylalanine structure, therefore an aniline group has a phenyl ring that is aromatic by nature. Foods high in protein are where tyrosine is found. The structure of Tyrosine is given as:

Hence, the correct option is C. Tyrosine.
Additional Information: The structure of the other three compounds are as follows:
The structure of Lysine:

The structure of Glycine:

The structure of Alanine:

Note: The thing to note here is that there are only three aromatic amino acids among the 20 amino acids. The remaining three are Glycine, Alanine and Lysine and they all form a straight chain and no aromatic rings are there in their structure.
Recently Updated Pages
Types of Solutions in Chemistry: Explained Simply

JEE General Topics in Chemistry Important Concepts and Tips

JEE Extractive Metallurgy Important Concepts and Tips for Exam Preparation

JEE Amino Acids and Peptides Important Concepts and Tips for Exam Preparation

JEE Atomic Structure and Chemical Bonding important Concepts and Tips

Electricity and Magnetism Explained: Key Concepts & Applications

Trending doubts
JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding How a Current Loop Acts as a Magnetic Dipole

Understanding Average and RMS Value in Electrical Circuits

Understanding Collisions: Types and Examples for Students

Ideal and Non-Ideal Solutions Explained for Class 12 Chemistry

Other Pages
JEE Advanced Weightage 2025 Chapter-Wise for Physics, Maths and Chemistry

JEE Main Participating Colleges 2026 - A Complete List of Top Colleges

Understanding Atomic Structure for Beginners

NCERT Solutions For Class 12 Chemistry Chapter 2 Solutions Hindi Medium (2025-26)

AssertionIn electrolytic refining of metal impure metal class 12 chemistry JEE_Main

CBSE Class 12 Chemistry Set 1 56/2/1 2025: Question Paper, Answers & Analysis




