
The unshared pair of electrons on a cyanide ion can acts as
A. Isocyanide centre
B. Amido centre
C. Cationic centre
D. Nucleophilic centre
Answer
232.8k+ views
Hint: Cyanide is a functional group possessing the formula of CN. There is a triple bond between carbon and the nitrogen atom. The acidic hydrolysis of cyanide gives carboxylic acid. Cyanide is an ambident nucleophile.
Complete Step by Step Solution:
The structure of the cyanide ion is,
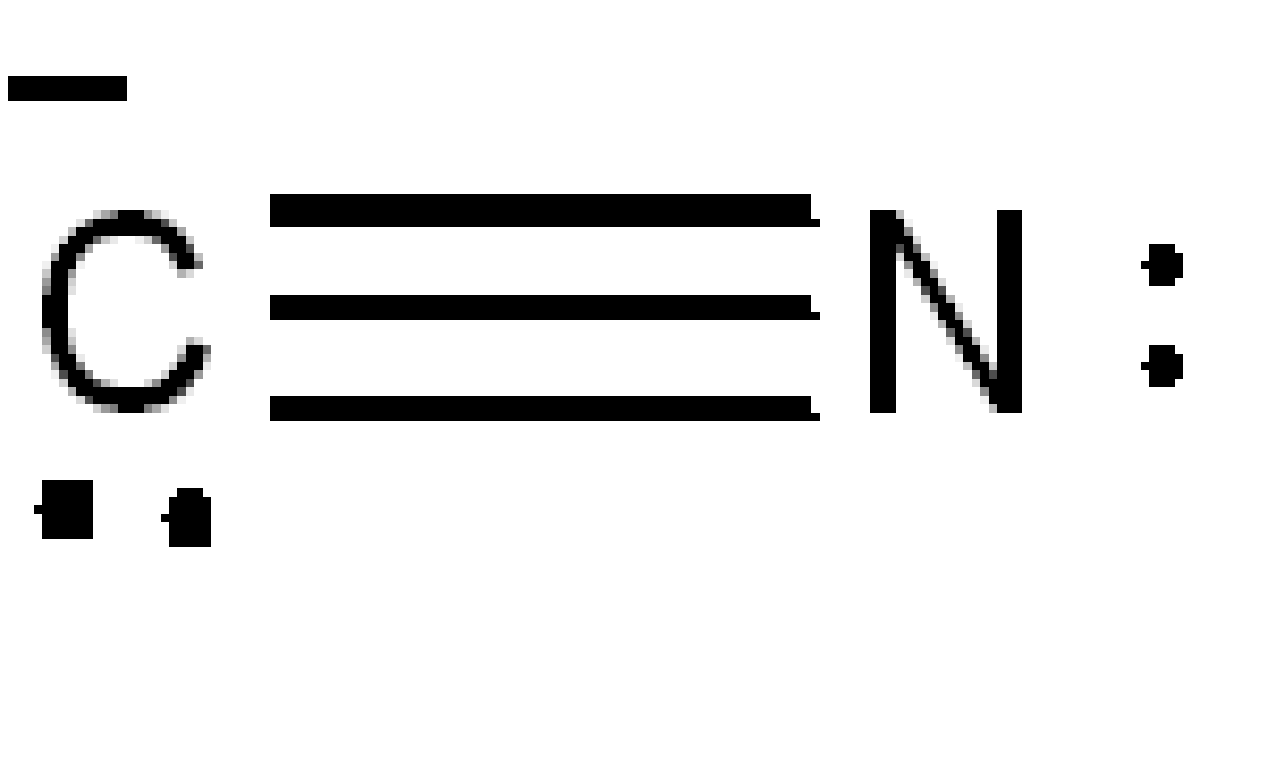
Image: Cyanide ion
Now, let's look at the resonance of the cyanide ion.
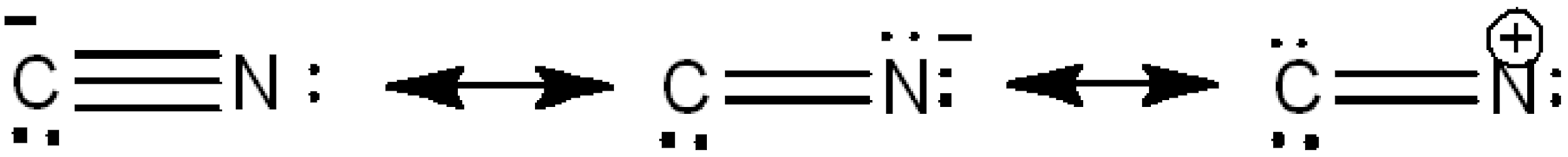
Image: Resonance of cyanide ion
As we can see, the cyanide ion has lone pair of electrons. So, it can attract positive charges. Therefore, cyanide ion acts as a ca cationic centre.Hence, option C is right.
Additional information/b>
Let's understand the nucleophilic characteristics of the cyanide ion. From the end of Carbon, the behavior of Cyanide ion (-CN-) as a nucleophile is stronger because it will result in the formation of C-C bond whose stability is more than the C-N bond.
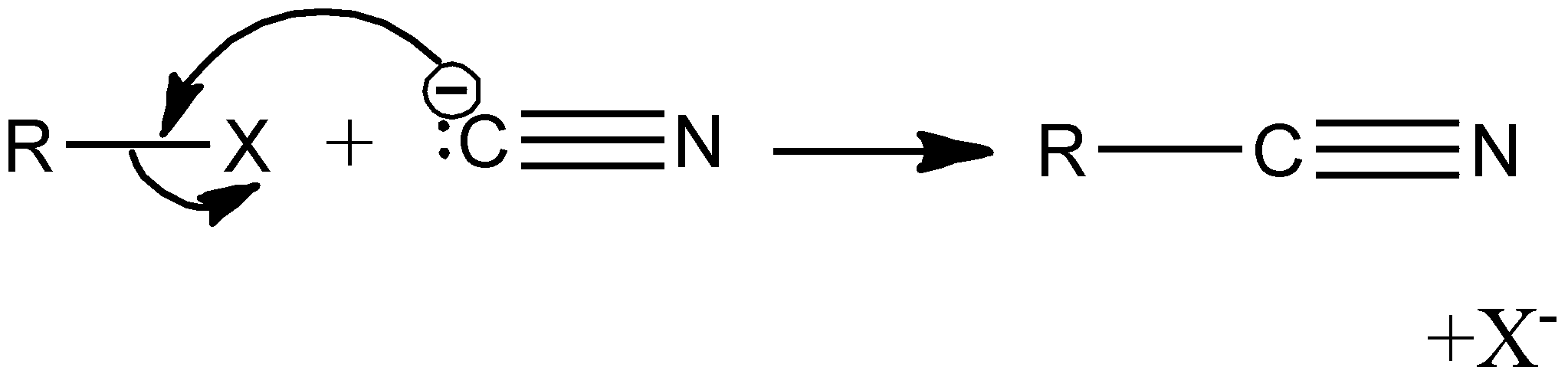
Image: Reaction of an alkyl halide with cyanide ion
Cyanide ion is an ambident nucleophile and reacts either through Carbon or Nitrogen atom. An ambident nucleophile is one in which either can happen through two sites of the same atom. For example, in the cyanide ion, the reaction happens through either C or N atom.
Note: It is to be noted both cyanide and isocyanide are two different functional groups. In cyanide, the reaction happens at the C atom, that is, bond formation takes place like (-CN). In isocyanide, the reaction happens at the N atom, that is, bond formation occurs as (R-NC).
Complete Step by Step Solution:
The structure of the cyanide ion is,

Image: Cyanide ion
Now, let's look at the resonance of the cyanide ion.
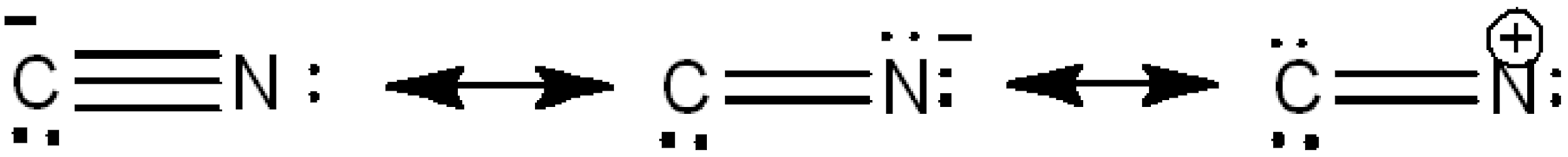
Image: Resonance of cyanide ion
As we can see, the cyanide ion has lone pair of electrons. So, it can attract positive charges. Therefore, cyanide ion acts as a ca cationic centre.Hence, option C is right.
Additional information/b>
Let's understand the nucleophilic characteristics of the cyanide ion. From the end of Carbon, the behavior of Cyanide ion (-CN-) as a nucleophile is stronger because it will result in the formation of C-C bond whose stability is more than the C-N bond.

Image: Reaction of an alkyl halide with cyanide ion
Cyanide ion is an ambident nucleophile and reacts either through Carbon or Nitrogen atom. An ambident nucleophile is one in which either can happen through two sites of the same atom. For example, in the cyanide ion, the reaction happens through either C or N atom.
Note: It is to be noted both cyanide and isocyanide are two different functional groups. In cyanide, the reaction happens at the C atom, that is, bond formation takes place like (-CN). In isocyanide, the reaction happens at the N atom, that is, bond formation occurs as (R-NC).
Recently Updated Pages
Know The Difference Between Fluid And Liquid

Types of Solutions in Chemistry: Explained Simply

Difference Between Crystalline and Amorphous Solid: Table & Examples

Hess Law of Constant Heat Summation: Definition, Formula & Applications

Disproportionation Reaction: Definition, Example & JEE Guide

JEE General Topics in Chemistry Important Concepts and Tips

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




