
The product formed when benzene is nitrated by fuming nitric acid is
A. m-dinitrobenzene
B. Nitrobenzene
C. sym-trinitrobenzene
D. None of these
Answer
233.1k+ views
Hint: Benzene is an electron dense species due to presence of pi electrons. It can donate its electron pair. On reaction with nitric acid it undergoes nitration reaction and forms nitrobenzene as a product.
Complete Step by Step Answer:
Benzene is a nucleophile due to the presence of high electron density. On nitration the electrophile nitro group can easily attack any position of benzene and form a product. Benzene on fuming with nitric acid means the reaction takes place in presence of water.
Water is a polar solvent which activates the benzene ring so much that on treatment with nitric acid in water the electrophile or the nitro group attacks the ortho, meta and one para positions at a time and gives sym-trinitrobenzene as a major product along with three moles of water.
The nitration reaction of benzene is as follows:
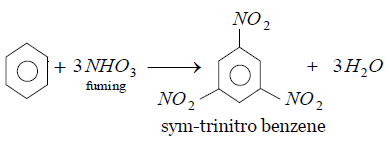
Thus the correct option is C.
Note: Nitration reaction is an aromatic electrophilic substitution reaction where an electrophile attacks a benzene ring. In the nitration of the benzene ring the nitro group acts as an electrophile due to low electron density and it easily attacks the more electron dense system that is the benzene ring to give product.
Complete Step by Step Answer:
Benzene is a nucleophile due to the presence of high electron density. On nitration the electrophile nitro group can easily attack any position of benzene and form a product. Benzene on fuming with nitric acid means the reaction takes place in presence of water.
Water is a polar solvent which activates the benzene ring so much that on treatment with nitric acid in water the electrophile or the nitro group attacks the ortho, meta and one para positions at a time and gives sym-trinitrobenzene as a major product along with three moles of water.
The nitration reaction of benzene is as follows:

Thus the correct option is C.
Note: Nitration reaction is an aromatic electrophilic substitution reaction where an electrophile attacks a benzene ring. In the nitration of the benzene ring the nitro group acts as an electrophile due to low electron density and it easily attacks the more electron dense system that is the benzene ring to give product.
Recently Updated Pages
Types of Solutions in Chemistry: Explained Simply

JEE General Topics in Chemistry Important Concepts and Tips

JEE Extractive Metallurgy Important Concepts and Tips for Exam Preparation

JEE Amino Acids and Peptides Important Concepts and Tips for Exam Preparation

JEE Atomic Structure and Chemical Bonding important Concepts and Tips

Electricity and Magnetism Explained: Key Concepts & Applications

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




