
The plastic household crockery is prepared by using
Option 1: Melamine and tetrafluoroethane
Option 2: Malonic acid and hexamethylenediamine
Option 3: Melamine and vinyl acetate
Option 4: Melamine and formaldehyde
Answer
233.4k+ views
Hint: A polymer is made up of small repeating units known as monomers. The size of a polymer is very large. The process of polymer formation from monomers is termed polymerization reaction.
Complete Step by Step Solution:
Let’s understand the substance that is used for making plastic household crockery. The plastic is made from melamine and formaldehyde. It is very tough and possesses very high durability. It is resistant to fire and heat. When two monomers namely melamine and formaldehyde undergo a condensation polymerization reaction, melamine formaldehyde forms. The fireproof property of this polymer is due to the release of nitrogen gas during the combustion process. This quality makes the plastic crockery unbreakable.
Now, we will see the reaction between melamine and formaldehyde. The reaction is,
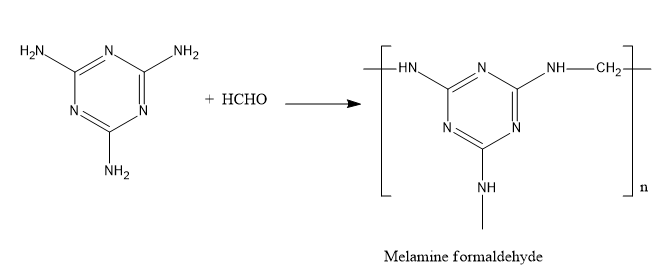
Fig: Reaction of melamine and formaldehyde
Therefore, the household crockery is made from melamine and formaldehyde.
Hence, option 4 is right.
Additional Information: In making household crockery, PTFE (Polytetrafluoroethylene) is used as a non-sticking coat because of its resistance to chemical reactions because of the presence of a carbon fluoride bond. The use of PTFE as a lubricant is also significant because of its capacity to reduce friction and energy efficiency.
Note: There are many useful applications of melamine formaldehyde polymer. It is a complex and interlinked polymer that makes it a hard and chemical-resistant material. Its use in the making of plywood, laminated countertops, and automotive surface coatings is significant.
Complete Step by Step Solution:
Let’s understand the substance that is used for making plastic household crockery. The plastic is made from melamine and formaldehyde. It is very tough and possesses very high durability. It is resistant to fire and heat. When two monomers namely melamine and formaldehyde undergo a condensation polymerization reaction, melamine formaldehyde forms. The fireproof property of this polymer is due to the release of nitrogen gas during the combustion process. This quality makes the plastic crockery unbreakable.
Now, we will see the reaction between melamine and formaldehyde. The reaction is,

Fig: Reaction of melamine and formaldehyde
Therefore, the household crockery is made from melamine and formaldehyde.
Hence, option 4 is right.
Additional Information: In making household crockery, PTFE (Polytetrafluoroethylene) is used as a non-sticking coat because of its resistance to chemical reactions because of the presence of a carbon fluoride bond. The use of PTFE as a lubricant is also significant because of its capacity to reduce friction and energy efficiency.
Note: There are many useful applications of melamine formaldehyde polymer. It is a complex and interlinked polymer that makes it a hard and chemical-resistant material. Its use in the making of plywood, laminated countertops, and automotive surface coatings is significant.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




