
The oxidation states of P atom in $POC{{l}_{3}},{{H}_{2}}P{{O}_{3}}$ and ${{H}_{4}}{{P}_{2}}{{O}_{6}}$ respectively are:
Answer
233.1k+ views
Hint: Draw the structures of $POC{{l}_{3}},{{H}_{2}}P{{O}_{3}}$ and ${{H}_{4}}{{P}_{2}}{{O}_{6}}$ .Keep in mind the valency of the P atom to avoid any mistakes. Try calculating oxidation state of P by trying to balance the positive and
the negative charge in the compound.
Complete step by step solution:
We will draw the structure for the three compounds given above to find the oxidation state.
Structure of $POC{{l}_{3}}$:
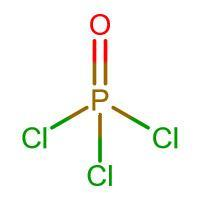
There are 5 bonds around the central P atom, hence the oxidation state of P is 5.
Structure of ${{H}_{2}}P{{O}_{3}}$:
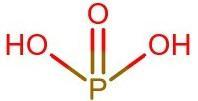
There are 4 bonds around the central P atom, hence the oxidation state of P is 4.
Structure of ${{H}_{4}}{{P}_{2}}{{O}_{6}}$:
In the above case, we consider the number of bonds around each P excluding the P-P bond as it is not counted when calculating the oxidation state. There are 4 bonds around each of the P atoms, hence the oxidation state is 4.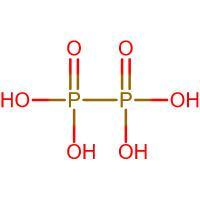
Therefore, the correct answer is option (A).
Additional information:
While calculating the oxidation state of an atom we count the number of bonds around the atom. In reality, we mean to count the number of heterogeneous bonds i.e. both the atoms are not identical. Homogenous bonds like P-P do not contribute to the oxidation state of the atom and hence it is not counted.
Note: While writing the structure of the compound, keep in mind the maximum valency the central atom can show and make the bonds accordingly. Do not count homogenous bonds while calculating the oxidation state of the central atom.
the negative charge in the compound.
Complete step by step solution:
We will draw the structure for the three compounds given above to find the oxidation state.
Structure of $POC{{l}_{3}}$:

There are 5 bonds around the central P atom, hence the oxidation state of P is 5.
Structure of ${{H}_{2}}P{{O}_{3}}$:

There are 4 bonds around the central P atom, hence the oxidation state of P is 4.
Structure of ${{H}_{4}}{{P}_{2}}{{O}_{6}}$:
In the above case, we consider the number of bonds around each P excluding the P-P bond as it is not counted when calculating the oxidation state. There are 4 bonds around each of the P atoms, hence the oxidation state is 4.

Therefore, the correct answer is option (A).
Additional information:
While calculating the oxidation state of an atom we count the number of bonds around the atom. In reality, we mean to count the number of heterogeneous bonds i.e. both the atoms are not identical. Homogenous bonds like P-P do not contribute to the oxidation state of the atom and hence it is not counted.
Note: While writing the structure of the compound, keep in mind the maximum valency the central atom can show and make the bonds accordingly. Do not count homogenous bonds while calculating the oxidation state of the central atom.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




