
The major product obtained on treatment of \[C{H_3}C{H_2}CH(F)C{H_3}\] with \[C{H_3}{O^ - }/C{H_3}OH\] is:
A. \[C{H_3}C{H_2}CH(OC{H_3})C{H_3}\]
B. \[C{H_3}CH = CHC{H_3}\]
C. \[C{H_3}C{H_2}CH = C{H_2}\]
D. \[C{H_3}C{H_2}C{H_2}C{H_2}OC{H_3}\]
Answer
233.1k+ views
Hint: Here, an alkyl halide is reacting with a methoxide ion (\[C{H_3}{O^ - }\] 9) in the presence of methanol (\[C{H_3}OH\]). The alkyl halide will undergo an elimination reaction called dehydrohalogenation. This elimination reaction will result in a mixture of products, only one of which will be the major product due to its stability.
Complete Step by Step Solution:
In the given question, we have been asked to predict the major product when 2-fluorobutane reacts with a methoxide ion (\[C{H_3}{O^ - }\] ) in the presence of methanol (\[C{H_3}OH\] ) as shown below:
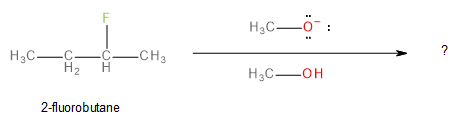
Image: Reaction in question
The methoxide ion has lone pairs of electrons that it can donate to an electron-deficient carbon atom and thus, act as a nucleophile. However, it does not act like a nucleophile. Instead, the methoxide ion prefers to attack a hydrogen atom of 2-fluorobutane and abstract it as a proton (\[{H^ + }\]). Thus, the methoxide ion acts as a base.
If the methoxide ion had acted like a nucleophile, it would have caused a nucleophilic substitution reaction of 2-fluorobutane. Since it acts as a base instead, the reaction that would occur here would be an elimination reaction. Elimination reactions can be either “unimolecular” (the E1 mechanism) or “bimolecular” (the E2 mechanism). Methoxide ion is a strong base; therefore, it would prefer the bimolecular elimination pathway (E2).
The E2 elimination mechanism, in this case, would involve the methoxide ion abstracting a beta-hydrogen and the leaving of the fluorine atom (as fluoride \[{F^ - }\] ), in a single step. This reaction is called dehydrohalogenation.
The product of the reaction would be a mixture of two alkenes, but-1-ene and but-2-ene as shown below:
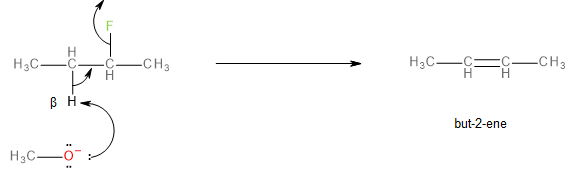
Image: Formation of but-2-ene
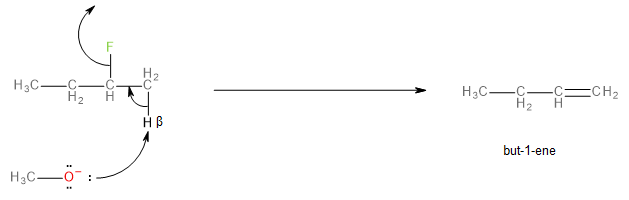
Image: Formation of but-1-ene
But-2-ene is called the Saytzeff product while but-1-ene is called the Hofmann product of the dehydrohalogenation reaction. Since the methoxide ion is less bulky, it is less sterically hindered. Less sterically hindered bases usually have the Saytzeff product as the major one and the Hofmann product the minor one. This is because the Saytzeff product in this reaction (but-2-ene) is more substituted than the Hofmann product (but-1-ene) which makes it more stable.
Thus, option B is correct.
Note: Students may be prone to mistaking the methoxide ion as a nucleophile, considering the reaction to be a nucleophilic substitution, and marking option A as their choice. They would be wrong if they did that. Nucleophilicity and basicity are not the same. Nucleophilicity refers to the ability of an electron-rich species to donate its lone pairs of electrons to electron-deficient species. Therefore, all nucleophiles are Lewis bases. But basicity refers to the ability of an electron-rich species to accept a proton. When an electron-rich species acts as an electron donor (i.e., a nucleophile), it causes substitution. When an electron-rich species acts as a proton acceptor (i.e., a base), it causes elimination. It is highly advised that students learn about the extent of nucleophilicities and basicities of different electron-rich species.
Complete Step by Step Solution:
In the given question, we have been asked to predict the major product when 2-fluorobutane reacts with a methoxide ion (\[C{H_3}{O^ - }\] ) in the presence of methanol (\[C{H_3}OH\] ) as shown below:

Image: Reaction in question
The methoxide ion has lone pairs of electrons that it can donate to an electron-deficient carbon atom and thus, act as a nucleophile. However, it does not act like a nucleophile. Instead, the methoxide ion prefers to attack a hydrogen atom of 2-fluorobutane and abstract it as a proton (\[{H^ + }\]). Thus, the methoxide ion acts as a base.
If the methoxide ion had acted like a nucleophile, it would have caused a nucleophilic substitution reaction of 2-fluorobutane. Since it acts as a base instead, the reaction that would occur here would be an elimination reaction. Elimination reactions can be either “unimolecular” (the E1 mechanism) or “bimolecular” (the E2 mechanism). Methoxide ion is a strong base; therefore, it would prefer the bimolecular elimination pathway (E2).
The E2 elimination mechanism, in this case, would involve the methoxide ion abstracting a beta-hydrogen and the leaving of the fluorine atom (as fluoride \[{F^ - }\] ), in a single step. This reaction is called dehydrohalogenation.
The product of the reaction would be a mixture of two alkenes, but-1-ene and but-2-ene as shown below:

Image: Formation of but-2-ene

Image: Formation of but-1-ene
But-2-ene is called the Saytzeff product while but-1-ene is called the Hofmann product of the dehydrohalogenation reaction. Since the methoxide ion is less bulky, it is less sterically hindered. Less sterically hindered bases usually have the Saytzeff product as the major one and the Hofmann product the minor one. This is because the Saytzeff product in this reaction (but-2-ene) is more substituted than the Hofmann product (but-1-ene) which makes it more stable.
Thus, option B is correct.
Note: Students may be prone to mistaking the methoxide ion as a nucleophile, considering the reaction to be a nucleophilic substitution, and marking option A as their choice. They would be wrong if they did that. Nucleophilicity and basicity are not the same. Nucleophilicity refers to the ability of an electron-rich species to donate its lone pairs of electrons to electron-deficient species. Therefore, all nucleophiles are Lewis bases. But basicity refers to the ability of an electron-rich species to accept a proton. When an electron-rich species acts as an electron donor (i.e., a nucleophile), it causes substitution. When an electron-rich species acts as a proton acceptor (i.e., a base), it causes elimination. It is highly advised that students learn about the extent of nucleophilicities and basicities of different electron-rich species.
Recently Updated Pages
JEE Main 2026 Session 2 Registration Open, Exam Dates, Syllabus & Eligibility

JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

Trending doubts
JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding Average and RMS Value in Electrical Circuits

Understanding Collisions: Types and Examples for Students

Ideal and Non-Ideal Solutions Explained for Class 12 Chemistry

Understanding Atomic Structure for Beginners

AssertionIn electrolytic refining of metal impure metal class 12 chemistry JEE_Main

Other Pages
JEE Advanced Weightage 2025 Chapter-Wise for Physics, Maths and Chemistry

NCERT Solutions For Class 12 Chemistry Chapter 2 Solutions Hindi Medium (2025-26)

CBSE Class 12 Chemistry Set 1 56/2/1 2025: Question Paper, Answers & Analysis

CBSE Class 12 Chemistry Question Paper Set 3 2025 with Answers

Inductive Effect and Its Role in Acidic Strength

Degree of Dissociation: Meaning, Formula, Calculation & Uses




