
The IUPAC name of the compound glycerine is:
A) 1,2,3-trihydroxypropane
B) 3-hydroxypentane-1,5-diol
C) 1,2,3-hydroxypropane
D) propane-1,2,3-triol
Answer
233.1k+ views
Hint: We know that IUPAC naming is the standard naming of organic compounds. There is a set of rules in naming of organic compounds. Identify the longest carbon chain and functional groups after drawing the structure.
Complete step-by-step solution:
Functional groups are made up of one or more atoms with distinctive chemical properties, independent of what is connected to them. The atoms of functional groups are bound by covalent bonds with each other and with the rest of the molecule.
The name of an organic compound has three parts, prefix, parent chain and suffix. The format of naming is ‘prefix +parent chain +suffix’. The longest chain of carbon atoms is the parent chain of the compound. Suffix denotes the name and position of functional groups and prefix denote the substituent and its position in the compound.
The structure of glycerine is,
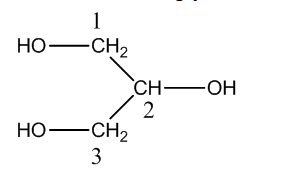
Now, we have to identify the parent chain in the glycerine. There are three carbon atoms in the parent chain and all bonds are single bonds. So, name of the parent chain is ‘propane’. The functional group –OH is present in all the three carbon atoms. So, suffix is ‘1,2,3-triol’ So, the IUPAC naming of glycerine is propan-1,2,3-triol.
Hence, correct option is D.
Note: The group with the formula OH is a hydroxyl group. This is made up of oxygen bound to hydrogen. Throughout organic chemistry, hydroxyl groups are found throughout alcohols and carboxylic acids. All the negatively charged ${\rm{ - O}}{{\rm{H}}^ - }$ anion, called hydroxide, and the stable radical ${\rm{ - OH}}$, known as hydroxyl radical, compose of an unbounded group of hydroxyl.
Complete step-by-step solution:
Functional groups are made up of one or more atoms with distinctive chemical properties, independent of what is connected to them. The atoms of functional groups are bound by covalent bonds with each other and with the rest of the molecule.
The name of an organic compound has three parts, prefix, parent chain and suffix. The format of naming is ‘prefix +parent chain +suffix’. The longest chain of carbon atoms is the parent chain of the compound. Suffix denotes the name and position of functional groups and prefix denote the substituent and its position in the compound.
The structure of glycerine is,

Now, we have to identify the parent chain in the glycerine. There are three carbon atoms in the parent chain and all bonds are single bonds. So, name of the parent chain is ‘propane’. The functional group –OH is present in all the three carbon atoms. So, suffix is ‘1,2,3-triol’ So, the IUPAC naming of glycerine is propan-1,2,3-triol.
Hence, correct option is D.
Note: The group with the formula OH is a hydroxyl group. This is made up of oxygen bound to hydrogen. Throughout organic chemistry, hydroxyl groups are found throughout alcohols and carboxylic acids. All the negatively charged ${\rm{ - O}}{{\rm{H}}^ - }$ anion, called hydroxide, and the stable radical ${\rm{ - OH}}$, known as hydroxyl radical, compose of an unbounded group of hydroxyl.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




