
The dehydrobromination of 2-bromobutane gives but-2-ene. The product is
A. Hofmann product
B. Saytzeff product
C. Hofmann - Saytzeff product
D. Markovnikov product
Answer
240.3k+ views
Hint: When a haloalkane reacts with an alcoholic potassium hydroxide (KOH) solution, an alkene is formed as the product. This reaction is called dehydrohalogenation.
Complete Step by Step Solution:
In dehydrohalogenation, a halogen atom and a hydrogen atom from the haloalkane get eliminated in the form of hydrogen halide.
It is an elimination reaction.
Alcoholic KOH is the concentrated alcoholic solution of KOH.
The carbon atom which carries the halogen atom is called α-carbon.
The carbon atom which holds the hydrogen atom is called β-carbon. As we notice, the hydrogen atom is lost from β-carbon, this reaction is also called the β-elimination reaction.
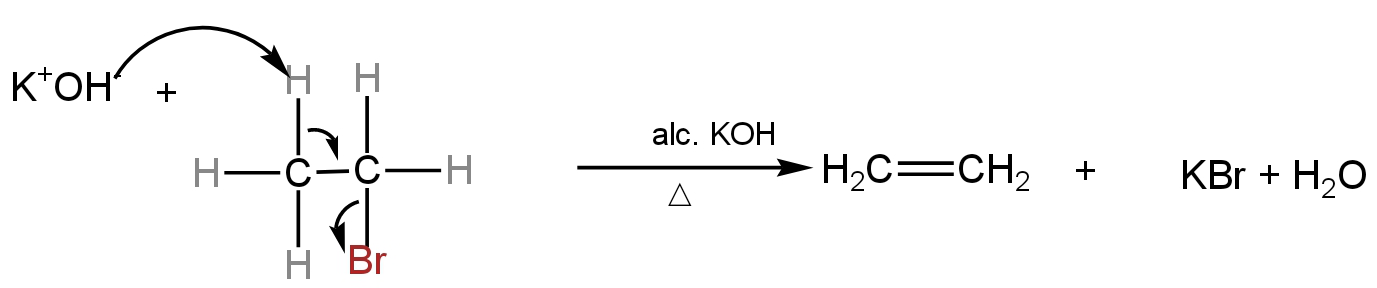
Image: Dehydrobromination of bromoethane forming ethene as the product.
We are given a compound 2-bromobutane which undergoes dehydrobromination giving but-2-ene.
A. Hoffman's product is formed as a result of Hofmann's elimination.
Hofmann elimination is an elimination reaction involving an amine establishing alkenes.
The insufficiently stable alkene is the Hofmann product.
In this reaction amine is not a reactant, so the product formed is not a Hofmann product.
B. In the given reaction, the halogen atom is present on a carbon atom within the chain.
Thus, the alkyl halide can undergo two different types of reactions depending on the two different types of β-hydrogens available.
According to Saytzeff's rule, in this type of reaction, the more highly substituted alkene, i.e, the alkene having a lesser number of hydrogen atoms on the doubly bonded carbon atoms is the major product.
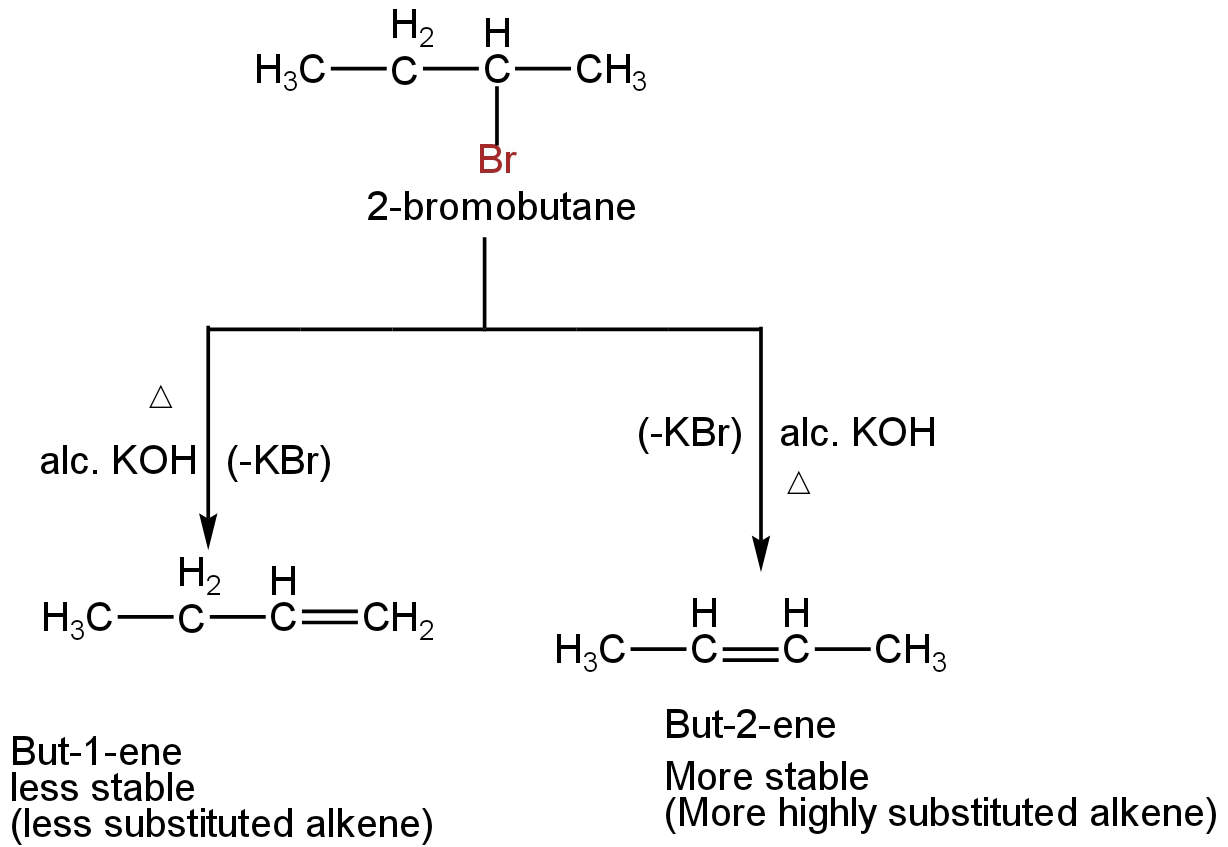
Image: Dehydrobromination of 2-bromobutane.
So, the product is Saytzeff's product.
C. The reaction does not have a Hofmann-saytzeff product.
D. Markovnikov's rule is applied during the reaction of a protic acid (HX) with an asymmetric alkene.
This reaction does not involve a protic acid.
So, the Markovnikov product is not formed.
So, option B is correct.
Note: In the given reaction, the halogen atom is present on a carbon atom within the chain due to which the alkyl halide can undergo two different types of reactions depending on the two different types of β-hydrogens available.
Here but-2-ene is the major product due to hyperconjugation.
Hyperconjugation or σ-conjugation or no-bond resonance is the delocalization of electrons with the bonds having mainly σ-character.
In this case, a sigma bond and adjacent pi-bond are involved in resonance.
In but-1-ene, the carbon atom attached to the double bond has only two hydrogen atoms.
So, only two hyperconjugative structures are possible.
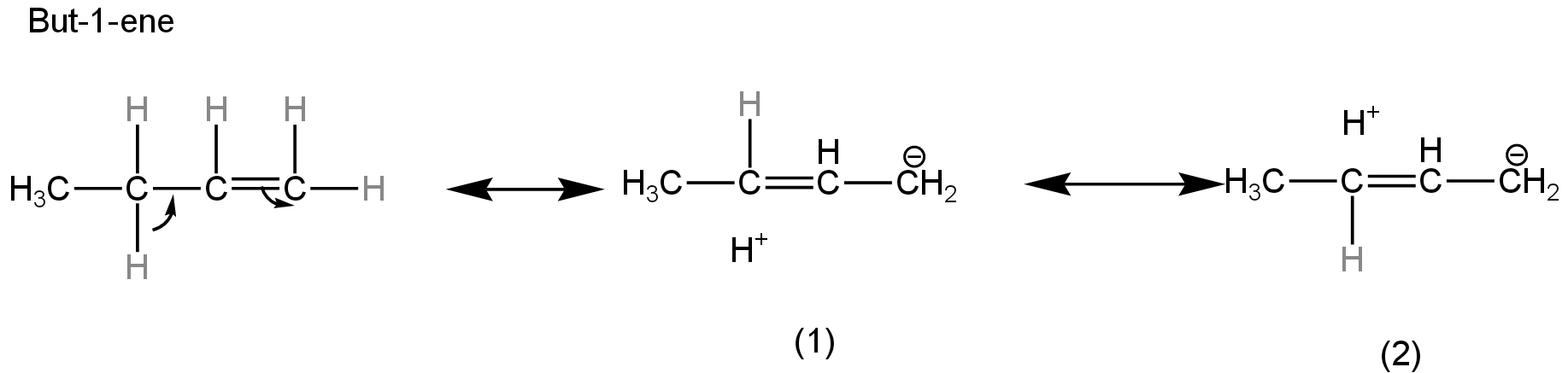
Image: Hyperconjugative structures of but-1ene.
In but-2-ene, we have 2 alkyl groups attached to the double bond. Six hydrogen atoms are attached to the double bond. So, six hyperconjugative structures are possible.
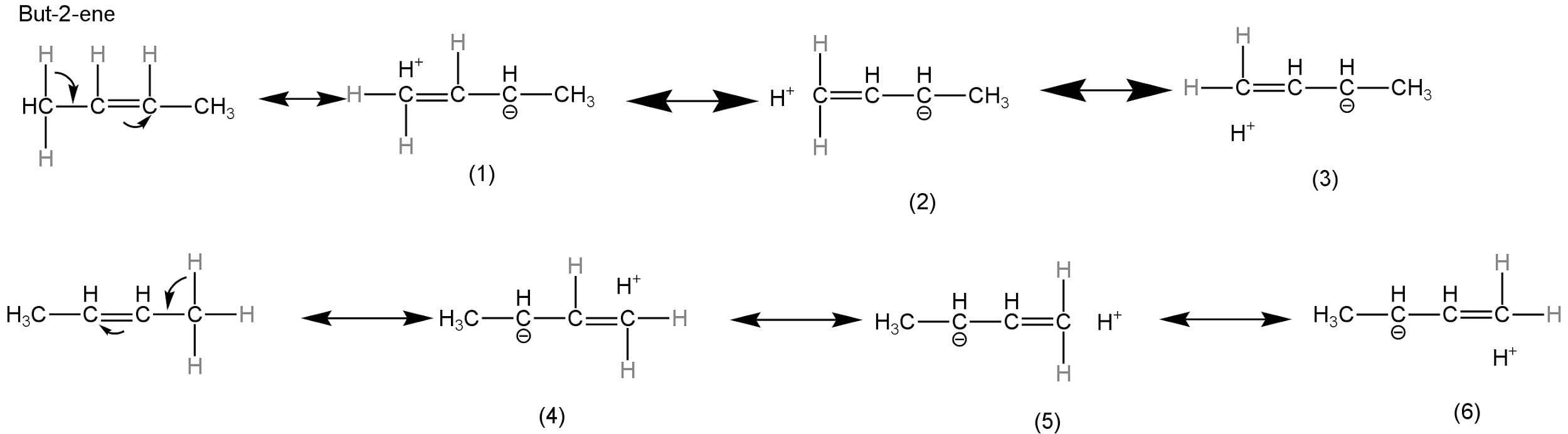
Image: Hyperconjugative structures of but-2-ene.
Hence, but-2-ene is more stable than but-1-ene.
Complete Step by Step Solution:
In dehydrohalogenation, a halogen atom and a hydrogen atom from the haloalkane get eliminated in the form of hydrogen halide.
It is an elimination reaction.
Alcoholic KOH is the concentrated alcoholic solution of KOH.
The carbon atom which carries the halogen atom is called α-carbon.
The carbon atom which holds the hydrogen atom is called β-carbon. As we notice, the hydrogen atom is lost from β-carbon, this reaction is also called the β-elimination reaction.

Image: Dehydrobromination of bromoethane forming ethene as the product.
We are given a compound 2-bromobutane which undergoes dehydrobromination giving but-2-ene.
A. Hoffman's product is formed as a result of Hofmann's elimination.
Hofmann elimination is an elimination reaction involving an amine establishing alkenes.
The insufficiently stable alkene is the Hofmann product.
In this reaction amine is not a reactant, so the product formed is not a Hofmann product.
B. In the given reaction, the halogen atom is present on a carbon atom within the chain.
Thus, the alkyl halide can undergo two different types of reactions depending on the two different types of β-hydrogens available.
According to Saytzeff's rule, in this type of reaction, the more highly substituted alkene, i.e, the alkene having a lesser number of hydrogen atoms on the doubly bonded carbon atoms is the major product.

Image: Dehydrobromination of 2-bromobutane.
So, the product is Saytzeff's product.
C. The reaction does not have a Hofmann-saytzeff product.
D. Markovnikov's rule is applied during the reaction of a protic acid (HX) with an asymmetric alkene.
This reaction does not involve a protic acid.
So, the Markovnikov product is not formed.
So, option B is correct.
Note: In the given reaction, the halogen atom is present on a carbon atom within the chain due to which the alkyl halide can undergo two different types of reactions depending on the two different types of β-hydrogens available.
Here but-2-ene is the major product due to hyperconjugation.
Hyperconjugation or σ-conjugation or no-bond resonance is the delocalization of electrons with the bonds having mainly σ-character.
In this case, a sigma bond and adjacent pi-bond are involved in resonance.
In but-1-ene, the carbon atom attached to the double bond has only two hydrogen atoms.
So, only two hyperconjugative structures are possible.

Image: Hyperconjugative structures of but-1ene.
In but-2-ene, we have 2 alkyl groups attached to the double bond. Six hydrogen atoms are attached to the double bond. So, six hyperconjugative structures are possible.

Image: Hyperconjugative structures of but-2-ene.
Hence, but-2-ene is more stable than but-1-ene.
Recently Updated Pages
Types of Solutions in Chemistry: Explained Simply

JEE Main Mock Test 2025-26: Principles Related To Practical

JEE Main 2025-26 Organic Compounds Containing Nitrogen Mock Test

JEE Main 2025-26 Mock Test: Organic Compounds Containing Oxygen

JEE Main 2025-26 Redox Reactions & Electro Mock Test

JEE Main Solutions Mock Test 1-2 (2025-26): Free Practice & Answers

Trending doubts
JEE Main 2026: Session 1 Results Out and Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

Ideal and Non-Ideal Solutions Explained for Class 12 Chemistry

JEE Main Participating Colleges 2026 - A Complete List of Top Colleges

Clemmensen and Wolff Kishner Reductions Explained for JEE & NEET

Degree of Dissociation: Meaning, Formula, Calculation & Uses

Understanding the Angle of Deviation in a Prism

Other Pages
CBSE Class 12 Chemistry Question Paper 2026 PDF Download (All Sets) with Answer Key

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The D And F Block Elements - 2025-26

JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 2 Electrochemistry - 2025-26

JEE Advanced 2026 - Exam Date (Released), Syllabus, Registration, Eligibility, Preparation, and More




