
The correct statement on the isomerism associated with the following complex ions is:
(a) ${{\left[ Ni{{\left( {{H}_{2}}O \right)}_{5}}N{{H}_{3}} \right]}^{2+}}$
(b) ${{\left[ Ni{{\left( {{H}_{2}}O \right)}_{4}}{{\left( N{{H}_{3}} \right)}_{2}} \right]}^{2+}}$
(c) ${{\left[ Ni{{\left( {{H}_{2}}O \right)}_{3}}{{\left( N{{H}_{3}} \right)}_{3}} \right]}^{2+}}$
(A) Both (a) and (b) show geometrical isomerism.
(B) (b) shows geometrical isomerism and (c) shows optical isomerism.
(C) (a) shows geometrical isomerism and (b) shows optical isomerism.
(D) Both (b) and (c) show geometrical isomerism.
Answer
232.8k+ views
Hint: Recollect the concept of coordination chemistry. Think about what is optical isomerism and what is geometrical isomerism. Draw the structures of the given three complexes and try to identify the isomerism present in them. Then choose the most suitable option as an answer.
Complete step by step solution:
- Stereoisomerism is the phenomena in which atoms have a different spatial arrangement. It is classified into two types: Optical isomerism and Geometrical isomerism.
- In optical isomerism, the two isomers are non-superimposable mirror images of each other. So, the point of contact between the metal and the ligands remains the same.
- In geometrical isomerism, the two isomers will have different arrangements of ligands around the central metal atom. This involves cis- and trans- isomers.
- Let’s draw the structure of the given coordination complexes.
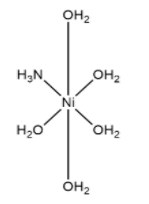
(a) ${{\left[ Ni{{\left( {{H}_{2}}O \right)}_{5}}N{{H}_{3}} \right]}^{2+}}$
(b) ${{\left[ Ni{{\left( {{H}_{2}}O \right)}_{4}}{{\left( N{{H}_{3}} \right)}_{2}} \right]}^{2+}}$
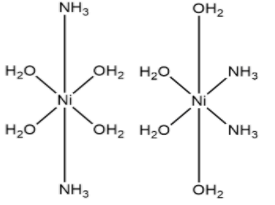
This complex shows geometrical isomerism and has trans and cis isomers which are shown above respectively.
(c) ${{\left[ Ni{{\left( {{H}_{2}}O \right)}_{3}}{{\left( N{{H}_{3}} \right)}_{3}} \right]}^{2+}}$
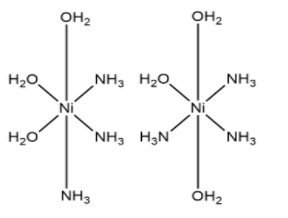
- This complex has facial and meridional, fac and mer isomers which are shown above. Therefore, this complex also shows geometrical isomerism.
- Therefore, the complexes (b) ${{\left[ Ni{{\left( {{H}_{2}}O \right)}_{4}}{{\left( N{{H}_{3}} \right)}_{2}} \right]}^{2+}}$ and (c) ${{\left[ Ni{{\left( {{H}_{2}}O \right)}_{3}}{{\left( N{{H}_{3}} \right)}_{3}} \right]}^{2+}}$ show geometrical isomerism.
Therefore, the correct answer is option (D).
Note: Remember fac isomer is the one in which ligands two ligands are in plane with metal and one ligand is out of plane. mer-isomer is the one in which three ligands and metal are in the same plane. cis-isomer is the one in which same ligands are adjacent to each other and trans isomer is the one in which same ligands are exactly opposite to each other.
Complete step by step solution:
- Stereoisomerism is the phenomena in which atoms have a different spatial arrangement. It is classified into two types: Optical isomerism and Geometrical isomerism.
- In optical isomerism, the two isomers are non-superimposable mirror images of each other. So, the point of contact between the metal and the ligands remains the same.
- In geometrical isomerism, the two isomers will have different arrangements of ligands around the central metal atom. This involves cis- and trans- isomers.
- Let’s draw the structure of the given coordination complexes.
(a) ${{\left[ Ni{{\left( {{H}_{2}}O \right)}_{5}}N{{H}_{3}} \right]}^{2+}}$

(b) ${{\left[ Ni{{\left( {{H}_{2}}O \right)}_{4}}{{\left( N{{H}_{3}} \right)}_{2}} \right]}^{2+}}$

This complex shows geometrical isomerism and has trans and cis isomers which are shown above respectively.
(c) ${{\left[ Ni{{\left( {{H}_{2}}O \right)}_{3}}{{\left( N{{H}_{3}} \right)}_{3}} \right]}^{2+}}$

- This complex has facial and meridional, fac and mer isomers which are shown above. Therefore, this complex also shows geometrical isomerism.
- Therefore, the complexes (b) ${{\left[ Ni{{\left( {{H}_{2}}O \right)}_{4}}{{\left( N{{H}_{3}} \right)}_{2}} \right]}^{2+}}$ and (c) ${{\left[ Ni{{\left( {{H}_{2}}O \right)}_{3}}{{\left( N{{H}_{3}} \right)}_{3}} \right]}^{2+}}$ show geometrical isomerism.
Therefore, the correct answer is option (D).
Note: Remember fac isomer is the one in which ligands two ligands are in plane with metal and one ligand is out of plane. mer-isomer is the one in which three ligands and metal are in the same plane. cis-isomer is the one in which same ligands are adjacent to each other and trans isomer is the one in which same ligands are exactly opposite to each other.
Recently Updated Pages
JEE Main 2026 Session 2 Registration Open, Exam Dates, Syllabus & Eligibility

JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

Trending doubts
JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

In Carius method of estimation of halogens 015g of class 11 chemistry JEE_Main

Understanding Average and RMS Value in Electrical Circuits

Understanding Collisions: Types and Examples for Students

Ideal and Non-Ideal Solutions Explained for Class 12 Chemistry

Understanding Atomic Structure for Beginners

Other Pages
JEE Advanced Weightage 2025 Chapter-Wise for Physics, Maths and Chemistry

NCERT Solutions For Class 11 Chemistry in Hindi Chapter 1 Some Basic Concepts of Chemistry (2025-26)

NCERT Solutions For Class 11 Chemistry in Hindi Chapter 8 Redox Reactions (2025-26)

An ideal gas is at pressure P and temperature T in class 11 chemistry JEE_Main

Inductive Effect and Its Role in Acidic Strength

Degree of Dissociation: Meaning, Formula, Calculation & Uses




