
The colour of blood is red due to the presence of:
(a) Magnesium
(b) Cytoglobin
(c) Haemoglobin
(d) All of the above
Answer
233.4k+ views
Hint: Red as a colour is mostly seen in compounds containing Iron-based compounds. With this in approach, try and think of the compounds in the options and their chemical constitution.
Complete step-by-step answer:
Before, answering this question, let us first break down the composition of blood and then get to the subject of answering this question.
- Blood is red because it is made up of cells that are red, which are called red blood cells. But, to understand why these cells are red you have to study them on a molecular level. Within the red blood cells there is a protein called haemoglobin. Each haemoglobin protein is made up of subunits called hemes, which are what give blood its red colour. More specifically, the hemes can bind iron molecules, and these iron molecules bind oxygen.
- The blood cells are red because of the interaction between iron and oxygen. (Even more specifically, it looks red because of how the chemical bonds between the iron and the oxygen reflect light.) And it's very important for blood to be able to carry oxygen because when blood flows through the lungs, the blood picks up oxygen, and the blood carries this oxygen to the rest of the body until the oxygen is all used up - the blood then returns to the lungs to get more oxygen.
Therefore, through this analysis, we can conclude that the answer to this question is c) Haemoglobin.
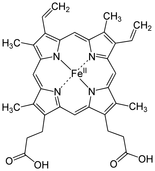
Note: A common misconception is that the red colour of blood is due to haemoglobin iron. The source of this myth likely originates from the fact that iron oxides (rust) have a reddish hue. In reality the reddish colour of haemoglobin derives from the porphyrin ring to which the iron is bound, not the iron itself.
Complete step-by-step answer:
Before, answering this question, let us first break down the composition of blood and then get to the subject of answering this question.
- Blood is red because it is made up of cells that are red, which are called red blood cells. But, to understand why these cells are red you have to study them on a molecular level. Within the red blood cells there is a protein called haemoglobin. Each haemoglobin protein is made up of subunits called hemes, which are what give blood its red colour. More specifically, the hemes can bind iron molecules, and these iron molecules bind oxygen.
- The blood cells are red because of the interaction between iron and oxygen. (Even more specifically, it looks red because of how the chemical bonds between the iron and the oxygen reflect light.) And it's very important for blood to be able to carry oxygen because when blood flows through the lungs, the blood picks up oxygen, and the blood carries this oxygen to the rest of the body until the oxygen is all used up - the blood then returns to the lungs to get more oxygen.
Therefore, through this analysis, we can conclude that the answer to this question is c) Haemoglobin.
Note: A common misconception is that the red colour of blood is due to haemoglobin iron. The source of this myth likely originates from the fact that iron oxides (rust) have a reddish hue. In reality the reddish colour of haemoglobin derives from the porphyrin ring to which the iron is bound, not the iron itself.

Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




