
The ABAB packing and ABCABC packing are respectively called as:
A. Hexagonal close packing (hcp) and cubic close packing (CCP)
B. Cubic close packing (ccp) and hexagonal close packing (HCP)
C. Body centred cubic (bcc) and hexagonal close packing (HCP)
D. Hexagonal close packing (hcp) and body centred cubic (BCC)
Answer
240.6k+ views
Hint: The different packing structures or types can be identified on the basis of the molecular arrangement in the given lattice. Each type of packing structure has a unique arrangement of atoms within them that can be understood by viewing the lattice from a side profile.
Complete Step-by-Step answer:
The spatial arrangements of atoms within a given lattice can be done in one dimension or two dimensions or even in 3 dimensions. Cubic close packing (ccp), hexagonal close packing (hcp) and body centred cubic (bcc) are all 3-dimensional spatial arrangements.
The spatial arrangements for the given packing structures are given as follows:
1. Hexagonal close packing
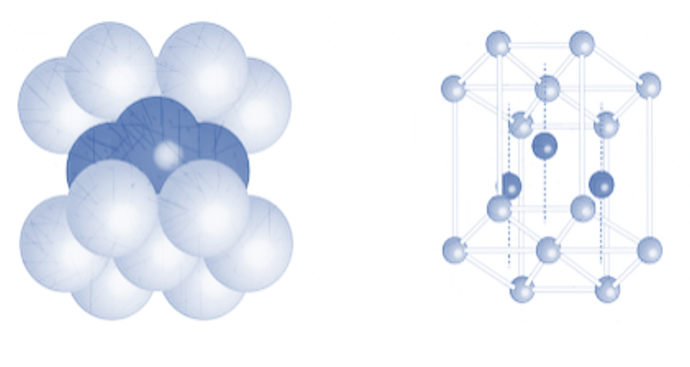
The arrangement in hexagonal packing as observed above is ABABAB
2. Cubic close packing
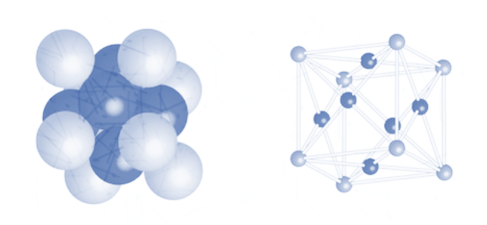
Face centred cubic packing is also known as cubic close packing. The arrangement in hexagonal packing as observed above is ABCABC
3. Body centred cubic packing
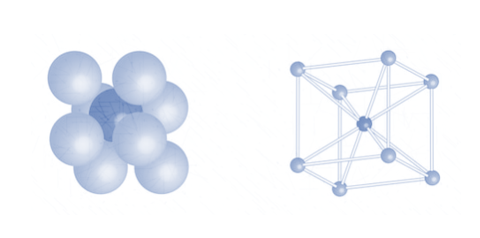
Since the body centric atoms are not in contact with any other atom within the same 2 – dimensional layer, it cannot be defined using a layer by layer interpretation.
Hence, the ABAB packing and ABCABC packing are respectively called as hexagonal close packing (hcp) and cubic close packing (ccp)
Hence, Option A is the correct option.
Note: The repetitive patterns of the different 2 - dimensional layers of the packing structures are named A, B, C and so on. So, each type of dimensional packing structure, has its own repetitive arrangements of the 2 - dimensional layers which can be represented in the above fashion.
Complete Step-by-Step answer:
The spatial arrangements of atoms within a given lattice can be done in one dimension or two dimensions or even in 3 dimensions. Cubic close packing (ccp), hexagonal close packing (hcp) and body centred cubic (bcc) are all 3-dimensional spatial arrangements.
The spatial arrangements for the given packing structures are given as follows:
1. Hexagonal close packing

The arrangement in hexagonal packing as observed above is ABABAB
2. Cubic close packing

Face centred cubic packing is also known as cubic close packing. The arrangement in hexagonal packing as observed above is ABCABC
3. Body centred cubic packing

Since the body centric atoms are not in contact with any other atom within the same 2 – dimensional layer, it cannot be defined using a layer by layer interpretation.
Hence, the ABAB packing and ABCABC packing are respectively called as hexagonal close packing (hcp) and cubic close packing (ccp)
Hence, Option A is the correct option.
Note: The repetitive patterns of the different 2 - dimensional layers of the packing structures are named A, B, C and so on. So, each type of dimensional packing structure, has its own repetitive arrangements of the 2 - dimensional layers which can be represented in the above fashion.
Recently Updated Pages
Types of Solutions in Chemistry: Explained Simply

JEE Main Mock Test 2025-26: Principles Related To Practical

JEE Main 2025-26 Organic Compounds Containing Nitrogen Mock Test

JEE Main 2025-26 Mock Test: Organic Compounds Containing Oxygen

JEE Main 2025-26 Redox Reactions & Electro Mock Test

JEE Main Solutions Mock Test 1-2 (2025-26): Free Practice & Answers

Trending doubts
JEE Main 2026: Session 1 Results Out and Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

Ideal and Non-Ideal Solutions Explained for Class 12 Chemistry

JEE Main Participating Colleges 2026 - A Complete List of Top Colleges

Clemmensen and Wolff Kishner Reductions Explained for JEE & NEET

Degree of Dissociation: Meaning, Formula, Calculation & Uses

Understanding the Angle of Deviation in a Prism

Other Pages
CBSE Class 12 Chemistry Question Paper 2026 PDF Download (All Sets) with Answer Key

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The D And F Block Elements - 2025-26

JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 2 Electrochemistry - 2025-26

JEE Advanced 2026 - Exam Date (Released), Syllabus, Registration, Eligibility, Preparation, and More




