
\[{\rm{A + CC}}{{\rm{l}}_{\rm{4}}}{\rm{ + KOH}} \to {\rm{Salicylic acid}}\]
'A' in the above reaction is
A.
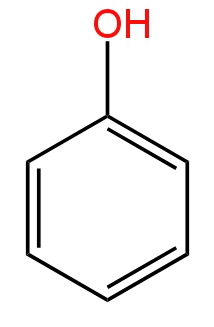
B.
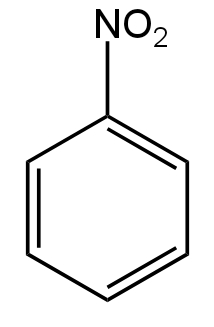
C.
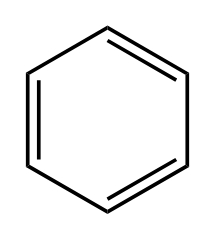
D.
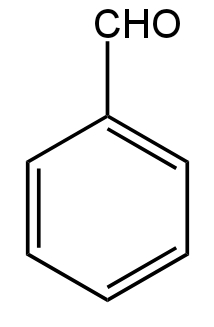
Answer
233.1k+ views
Hint: Treatment of phenol with carbon tetrachloride in the presence of aqueous sodium hydroxide gives salicylic acid as the major product. It is called Reimer-Tieman reaction.
Complete Step by Step Solution:
When phenol is treated with carbon tetrachloride in the presence of aqueous sodium or potassium hydroxide, salicylic acid is formed.
This reaction occurs at 340 K temperature.
The IUPAC name of Salicylic acid is 2-hydroxybenzoic acid.
Mechanism
This is an electrophilic substitution reaction.
Step-1
Phenol removes its proton when treated with sodium hydroxide producing sodium phenoxide.
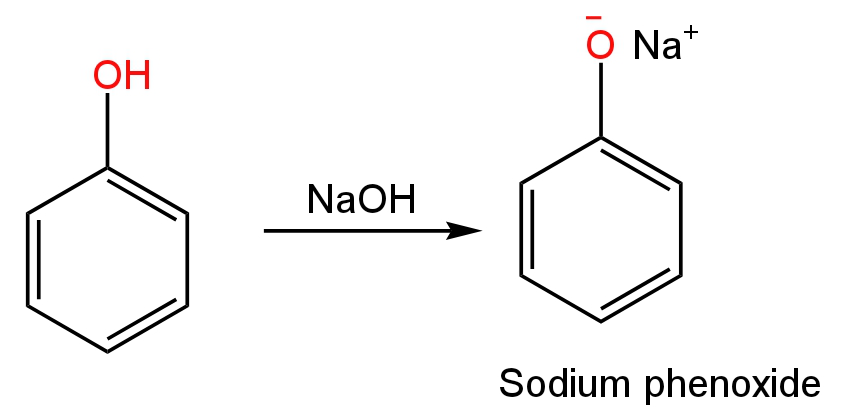
Image: Formation of sodium phenoxide ion from phenol.
Then the carbon tetrachloride attacks at the ortho position of the benzene ring.
The three chlorine atoms get replaced by three hydroxide ions when treated with sodium hydroxide.
A molecule of water gets removed from the newly formed product.
Treatment of aqueous sodium hydroxide and dilute hydrochloric acid leads to the formation of 2-Hydroxybenzoic acid.
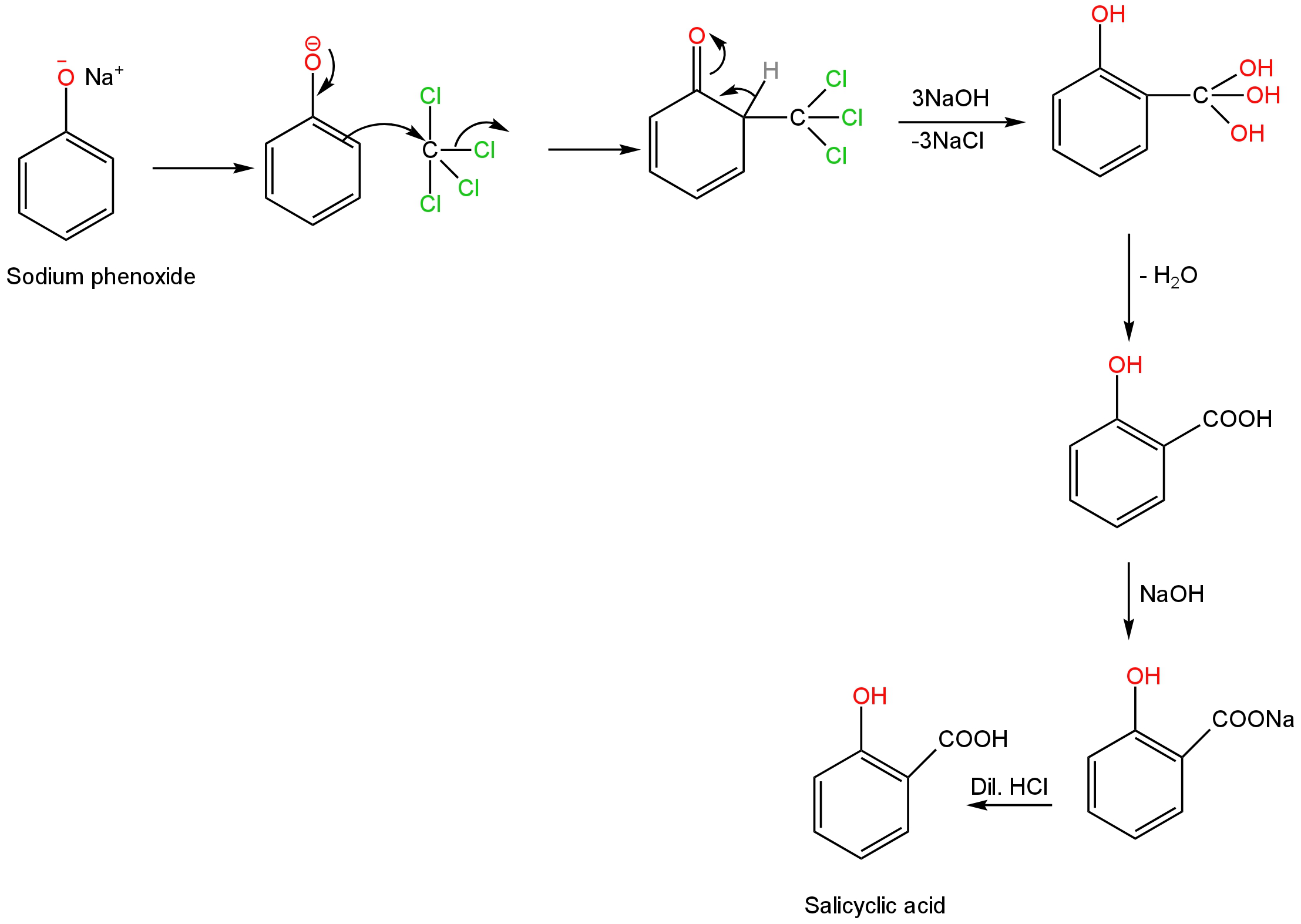
Image: Electrophilic substitution of phenol leading to the formation of salicylic acid.
So, option A is correct.
Additional Information: Treatment of phenol with chloroform in the presence of aqueous sodium or potassium hydroxide at 340 K followed by hydrolysis of the resulting product gives 2-hydroxybenzaldehyde or salicylaldehyde as the major product.
Dichlorocarbene is generated as an electrophile in this type of reaction.
Note: Reimer-Tieman reaction is used for the production of salicylic acid and salicylaldehyde.
For salicylic acid, carbon tetrachloride is used and for salicylaldehyde, chloroform is used.
Complete Step by Step Solution:
When phenol is treated with carbon tetrachloride in the presence of aqueous sodium or potassium hydroxide, salicylic acid is formed.
This reaction occurs at 340 K temperature.
The IUPAC name of Salicylic acid is 2-hydroxybenzoic acid.
Mechanism
This is an electrophilic substitution reaction.
Step-1
Phenol removes its proton when treated with sodium hydroxide producing sodium phenoxide.

Image: Formation of sodium phenoxide ion from phenol.
Then the carbon tetrachloride attacks at the ortho position of the benzene ring.
The three chlorine atoms get replaced by three hydroxide ions when treated with sodium hydroxide.
A molecule of water gets removed from the newly formed product.
Treatment of aqueous sodium hydroxide and dilute hydrochloric acid leads to the formation of 2-Hydroxybenzoic acid.

Image: Electrophilic substitution of phenol leading to the formation of salicylic acid.
So, option A is correct.
Additional Information: Treatment of phenol with chloroform in the presence of aqueous sodium or potassium hydroxide at 340 K followed by hydrolysis of the resulting product gives 2-hydroxybenzaldehyde or salicylaldehyde as the major product.
Dichlorocarbene is generated as an electrophile in this type of reaction.
Note: Reimer-Tieman reaction is used for the production of salicylic acid and salicylaldehyde.
For salicylic acid, carbon tetrachloride is used and for salicylaldehyde, chloroform is used.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




