
What is the product of the reaction of nitrous acid with aliphatic primary amine in the cold?
(A) A diazonium salt
(B) An alcohol
(C) A nitrite
(D) A dye
Answer
239.4k+ views
Hint: Amines are the compounds that contain an $N{{H}_{2}}$functional group in their structure. These are basic in nature as they contain nitrogen with a lone pair of electrons. These compounds are sensitive to pyrolysis when the $N{{H}_{2}}$ group is present.
Complete Step by Step Solution:
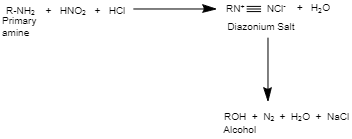
Reaction of $HN{{O}_{2}}$ with aliphatic amines, also known as the nitrous acid test, is an important test for distinguishing primary, secondary, and tertiary amines. When nitrous acid $HN{{O}_{2}}$ ) reacts with aliphatic primary amine in the cold, it first processes a diazonium salt, which is unstable. The diazonium salt then decomposes to alcohol along with the evolution of nitrogen gas (${{N}_{2}}$ ) and water.
Correct option: (B) An alcohol.
Additional information: At room temperature, primary amines are liquid, whereas secondary and tertiary amines are solid. Treatment of nitrous acid ($HN{{O}_{2}}$ ) with secondary amines gives an insoluble oil layer of nitrosamine; with tertiary amines, it gives a clear solution of quaternary ammonium salts.
Note: The nitrous acid test is used as a test for amines in organic chemistry to identify whether the compound has an amino functional group present or not. The primary amine can further be identified with the carbylamine test, in which amines are treated with chloroform in the presence of alkali. An alkyl isocyanide ($RNC$ ) is formed in this process. Both aliphatic and aromatic amines can give this test.
Complete Step by Step Solution:
Reaction of $HN{{O}_{2}}$ with aliphatic amines, also known as the nitrous acid test, is an important test for distinguishing primary, secondary, and tertiary amines. When nitrous acid $HN{{O}_{2}}$ ) reacts with aliphatic primary amine in the cold, it first processes a diazonium salt, which is unstable. The diazonium salt then decomposes to alcohol along with the evolution of nitrogen gas (${{N}_{2}}$ ) and water.

Correct option: (B) An alcohol.
Additional information: At room temperature, primary amines are liquid, whereas secondary and tertiary amines are solid. Treatment of nitrous acid ($HN{{O}_{2}}$ ) with secondary amines gives an insoluble oil layer of nitrosamine; with tertiary amines, it gives a clear solution of quaternary ammonium salts.
Note: The nitrous acid test is used as a test for amines in organic chemistry to identify whether the compound has an amino functional group present or not. The primary amine can further be identified with the carbylamine test, in which amines are treated with chloroform in the presence of alkali. An alkyl isocyanide ($RNC$ ) is formed in this process. Both aliphatic and aromatic amines can give this test.
Recently Updated Pages
Types of Solutions in Chemistry: Explained Simply

JEE Main Mock Test 2025-26: Principles Related To Practical

JEE Main 2025-26 Organic Compounds Containing Nitrogen Mock Test

JEE Main 2025-26 Mock Test: Organic Compounds Containing Oxygen

JEE Main 2025-26 Redox Reactions & Electro Mock Test

JEE Main Solutions Mock Test 1-2 (2025-26): Free Practice & Answers

Trending doubts
JEE Main 2026: Session 1 Results Out and Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

Ideal and Non-Ideal Solutions Explained for Class 12 Chemistry

JEE Main Participating Colleges 2026 - A Complete List of Top Colleges

Understanding the Angle of Deviation in a Prism

Understanding Electromagnetic Waves and Their Importance

Hybridisation in Chemistry – Concept, Types & Applications

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

JEE Advanced 2026 - Exam Date (Released), Syllabus, Registration, Eligibility, Preparation, and More

NCERT Solutions For Class 12 Chemistry Chapter 2 Electrochemistry - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The D And F Block Elements - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules - 2025-26




