
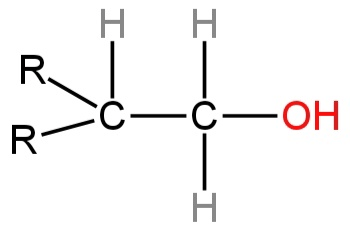
Product A in the following reaction is
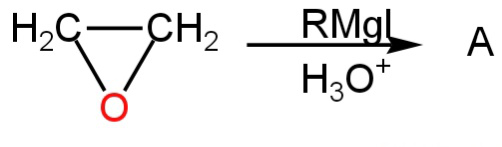
A.
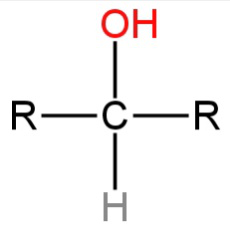
B.
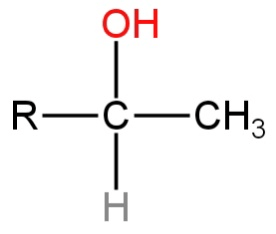
C.
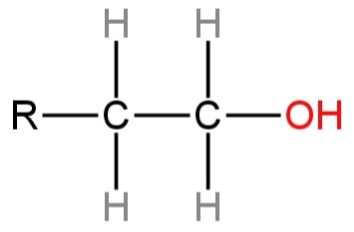
D.
Answer
233.1k+ views
Hint: C2H4O is ethylene oxide. It is an epoxide. Epoxides are ethers having the oxygen atom in a three-membered ring. Epoxides are also called oxiranes. RMgI i.e, alkyl magnesium iodide called Grignard's reagent.
Complete Step by Step Solution:
- Ethylene oxide is a strained ring, it readily undergoes several additional reactions which result in ring-opening.
- RMgI is an organometallic compound. An organometallic compound is defined as a compound that contains a direct carbon-metal bond.
- RMgX is Grignard's reagent. It is prepared by the action of alkyl halides on magnesium metal in the presence of dry ether.
- The C-Mg bond in Grignard reagents is polar. The carbon atom is additionally electronegative than magnesium.
- The electrons of the C-Mg bond are attracted to the carbon atom. So, the carbon atom is partially negatively charged, and the magnesium atom has a partial positive charge.
- The alkyl groups in Grignard reagents are electron-rich and can work as nucleophiles.
- These alkyl groups would strike polar molecules at the area of insufficient electron density. Thus, Grignard reagents typically undergo nucleophilic substitution and nucleophilic addition reactions.
- In ethylene oxide, on the other hand, oxygen is much more electronegative than carbon or hydrogen.
- The C-O and O-H bonds are all polar. Grignard reagents are powerful nucleophiles and can react with the carbon atom of epoxides. The reaction results in ring-opening and the formation of alcohol.
This reaction happens in two steps.
Step-1
The attack of the RMgI on ethylene oxide leads to the formation of an additional product that is unstable.
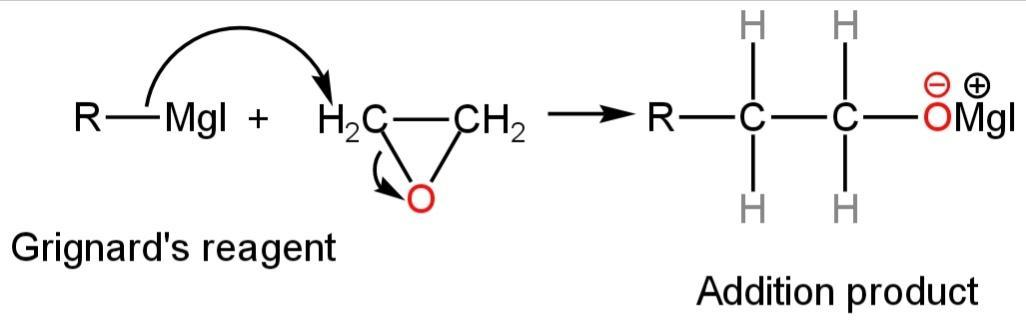
Image: Formation of additional products.
Step-2
The additional product undergoes hydrolysis to form alcohol.
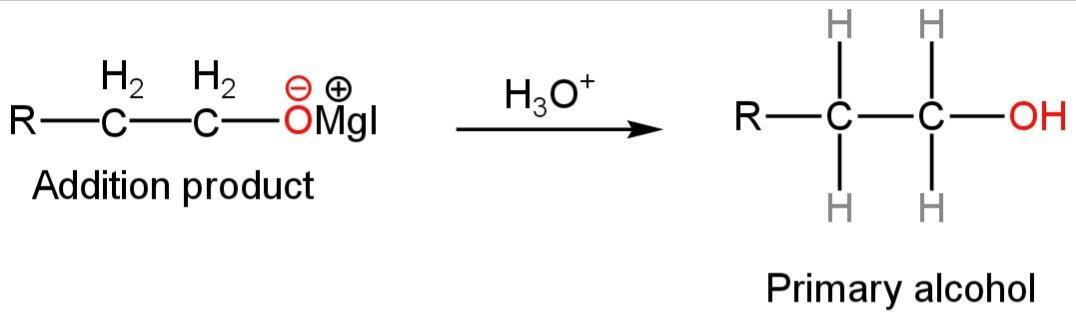
So, ethylene oxide in reaction with RMgI forms primary alcohol.
So, option A is correct.
Note: It must be noted that the ease of reaction of epoxide with RMgI increases with the increase in the ionic character of the C-Mg bond. In the preparation of Grignard reagents, a solution of the alkyl halide is dipped in dry ether into the reaction flask comprising magnesium ribbon suspended in dry ether. Dry ether is utilised as moisture may react with Grignard's reagent to form alkane. Thus, moisture or impurities discourage the yield of Grignard's reagent.
Complete Step by Step Solution:
- Ethylene oxide is a strained ring, it readily undergoes several additional reactions which result in ring-opening.
- RMgI is an organometallic compound. An organometallic compound is defined as a compound that contains a direct carbon-metal bond.
- RMgX is Grignard's reagent. It is prepared by the action of alkyl halides on magnesium metal in the presence of dry ether.
- The C-Mg bond in Grignard reagents is polar. The carbon atom is additionally electronegative than magnesium.
- The electrons of the C-Mg bond are attracted to the carbon atom. So, the carbon atom is partially negatively charged, and the magnesium atom has a partial positive charge.
- The alkyl groups in Grignard reagents are electron-rich and can work as nucleophiles.
- These alkyl groups would strike polar molecules at the area of insufficient electron density. Thus, Grignard reagents typically undergo nucleophilic substitution and nucleophilic addition reactions.
- In ethylene oxide, on the other hand, oxygen is much more electronegative than carbon or hydrogen.
- The C-O and O-H bonds are all polar. Grignard reagents are powerful nucleophiles and can react with the carbon atom of epoxides. The reaction results in ring-opening and the formation of alcohol.
This reaction happens in two steps.
Step-1
The attack of the RMgI on ethylene oxide leads to the formation of an additional product that is unstable.

Image: Formation of additional products.
Step-2
The additional product undergoes hydrolysis to form alcohol.

So, ethylene oxide in reaction with RMgI forms primary alcohol.
So, option A is correct.
Note: It must be noted that the ease of reaction of epoxide with RMgI increases with the increase in the ionic character of the C-Mg bond. In the preparation of Grignard reagents, a solution of the alkyl halide is dipped in dry ether into the reaction flask comprising magnesium ribbon suspended in dry ether. Dry ether is utilised as moisture may react with Grignard's reagent to form alkane. Thus, moisture or impurities discourage the yield of Grignard's reagent.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




