
Molecular weight of protein is:
A. > 12000
B. < 6000
C. < 12000
D. 600 – 3000
Answer
233.1k+ views
Hint: As protein made up of a hundred to a thousand of smaller structural units which is called amino acid. There are majorly 20 amino acids which are arranged in different manners to generate a protein. Molecular weight of proteins can be calculated by sum of individual weight of all amino acids which are used to build the protein.
Complete Step by Step Solution:
Amino acid, its name itself suggesting that amino acid is a combination of amino group and acid (carboxylic acid) such as
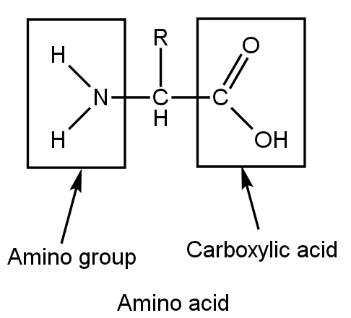
This type of amino acid combines with one or more other amino acids through peptide linkage. If there is less number of peptide linkage then it is called oligopeptides whereas if large number of Question:
For proteins which are polypeptides will consist 100 to 1000 of amino acids which together give average molecular weight greater than 1000 to 10,000 and can cross a million value. For sake of convenience, 110 Dalton or 110 amu is the average weight of single amino acid. After multiplying this value of weight to the total number of amino acids present in protein, give the average molecular weight of that protein.
So, molecular weight of protein must be greater than 12,000, thus, the correct option is A.
Note: It is important to note that we can calculate average molecular weight of protein because of the presence of many other variable groups along with carbon, nitrogen, carbonyl group, hydrogen and Sulphur and also due to the complexity of polypeptides.
Complete Step by Step Solution:
Amino acid, its name itself suggesting that amino acid is a combination of amino group and acid (carboxylic acid) such as

This type of amino acid combines with one or more other amino acids through peptide linkage. If there is less number of peptide linkage then it is called oligopeptides whereas if large number of Question:
For proteins which are polypeptides will consist 100 to 1000 of amino acids which together give average molecular weight greater than 1000 to 10,000 and can cross a million value. For sake of convenience, 110 Dalton or 110 amu is the average weight of single amino acid. After multiplying this value of weight to the total number of amino acids present in protein, give the average molecular weight of that protein.
So, molecular weight of protein must be greater than 12,000, thus, the correct option is A.
Note: It is important to note that we can calculate average molecular weight of protein because of the presence of many other variable groups along with carbon, nitrogen, carbonyl group, hydrogen and Sulphur and also due to the complexity of polypeptides.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




