
What is the Lintz-Dusenverfahren (LD) process?
A. It is metallurgical process in the manufacturing of copper to oxidise impurities
B. Type of zone refining to remove impurities from metals
C. It is pyrometallurgical process in the manufacturing of steel to oxidise impurities
D. None of these
Answer
232.8k+ views
Hint: LD is named after the two places of Austria namely, Linz and Donawitz where the process was first formed. It is a refining process which is done through an LD convertor or LD vessel. LD is also known as Basic Oxygen Process.
Complete step-by-step answer:
LD converter has a shape similar to a vessel with a height of around 10m. It is welded by steel plates and has a permanent lining and a working lining, made of magnesite bricks and dolomite bricks respectively. It also has an oxygen lance made up of concentric steel tubes with a tip of copper.
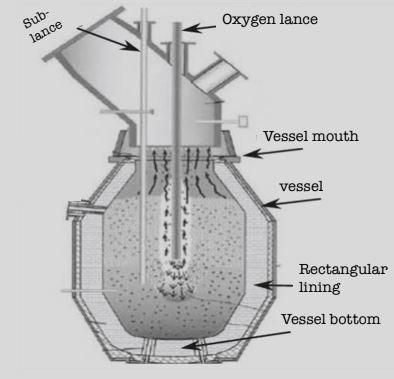
Now, let’s understand the process through the following steps:
Charging: Five materials are required for this purpose. Firstly, scrap takes up a quarter of metal charge in the LD process, it helps to utilize the excess heat energy and acts as a coolant during the process of refining. Hot metals like iron, carbon and silicon are also helpful in the process. While lime and dolomite are used as primary fluxes. Coolants like limestone, sponge iron, and scrap are used when there is excessive heating. Pure oxygen serves as a refining agent here.
Blowing: Once the charging is done then the converter is rotated upright in the vertical position and the lance is lowered to the position of blowing. \[{O_2}\]is then turned on for around 20 minutes, at a pressure of 9 to 11 atoms that raises the temperature and impurities are burned off.
Sampling: for analysis, slag and metal samples can be taken and the temperature of the bath needs to be measured by immersion of thermocouple.
Tapping: the molten steel is tapped in the ladle if the tapping temperature is in the required range. Ladle is used to make deoxidizers and alloying additions. It has a tap-to-tap time of 40-50mins.
Slag off: after tapping steel into the ladle and putting the vessel upside down tapped the remaining slag into the slag pot.
It has many advantages such as it is ten times faster than the open hearth process and does not use any external source of heat or fuel. It produces steel which has low sulphur and phosphorus content from raw materials and uses pure oxygen to eliminate the harmful effects of nitrogen.
Hence, the correct option is (C).
Note: There are some disadvantages too such as the charge must include a sufficient quantity of molten pig so as to minimize the amount of slag used. In this, steel wastage due to splashes by oxygen lancing is high and insufficient depth of penetration of oxygen results in thermal gradient.
Complete step-by-step answer:
LD converter has a shape similar to a vessel with a height of around 10m. It is welded by steel plates and has a permanent lining and a working lining, made of magnesite bricks and dolomite bricks respectively. It also has an oxygen lance made up of concentric steel tubes with a tip of copper.

Now, let’s understand the process through the following steps:
Charging: Five materials are required for this purpose. Firstly, scrap takes up a quarter of metal charge in the LD process, it helps to utilize the excess heat energy and acts as a coolant during the process of refining. Hot metals like iron, carbon and silicon are also helpful in the process. While lime and dolomite are used as primary fluxes. Coolants like limestone, sponge iron, and scrap are used when there is excessive heating. Pure oxygen serves as a refining agent here.
Blowing: Once the charging is done then the converter is rotated upright in the vertical position and the lance is lowered to the position of blowing. \[{O_2}\]is then turned on for around 20 minutes, at a pressure of 9 to 11 atoms that raises the temperature and impurities are burned off.
Sampling: for analysis, slag and metal samples can be taken and the temperature of the bath needs to be measured by immersion of thermocouple.
Tapping: the molten steel is tapped in the ladle if the tapping temperature is in the required range. Ladle is used to make deoxidizers and alloying additions. It has a tap-to-tap time of 40-50mins.
Slag off: after tapping steel into the ladle and putting the vessel upside down tapped the remaining slag into the slag pot.
It has many advantages such as it is ten times faster than the open hearth process and does not use any external source of heat or fuel. It produces steel which has low sulphur and phosphorus content from raw materials and uses pure oxygen to eliminate the harmful effects of nitrogen.
Hence, the correct option is (C).
Note: There are some disadvantages too such as the charge must include a sufficient quantity of molten pig so as to minimize the amount of slag used. In this, steel wastage due to splashes by oxygen lancing is high and insufficient depth of penetration of oxygen results in thermal gradient.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




