
In presence of \[AlC{l_3}\], benzene and n - propyl bromide react in Friedel - Craft's reaction to form:
A. N-propyl benzene
B. 1,2-Dinormal propyl benzene
C. 1,4-Dinormal propyl benzene
D. Isopropyl benzene
Answer
233.1k+ views
Hint: Friedel-Crafts reaction in consideration here is an alkylation reaction. It proceeds via an electrophilic aromatic substitution mechanism where the n-propyl bromide provides the electrophile intermediate.
Complete Step by Step Solution:
Friedel-Crafts reactions are a set of reactions which involve the attaching of a substituent to an aromatic ring. Friedel-Crafts reactions are of two types, alkylation reactions, in which an alkyl group (\[ - R\]) gets attached to a ring, and acylation reactions involving the attaching of an acyl group (\[ - RC = O\]) to a ring. In this reaction, the catalyst employed is aluminium chloride (\[AlC{l_3}\]) which is a strong Lewis acid. The alkylating agents have traditionally been alkyl halides.
Let’s have a look at how benzene and n-propyl bromide undergo Friedel-Craft’s reaction in presence of aluminium chloride.
In the first step, the n-propyl bromide loses its bromine atom with the help of aluminium chloride as shown. Since aluminium chloride is a strong Lewis acid, it accepts electrons from the bromine atom of n-propyl bromide, and it leads to the formation of an n-propyl carbocation.
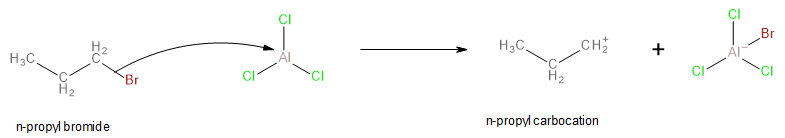
Image: Formation of n-propyl carbocation
The n-propyl carbocation formed is primary. We know that primary carbocations are the least stable (order of stability of carbocations: \[1^\circ < 2^\circ < 3^\circ \]). Since secondary carbocations are more stable, if the n-propyl carbocation can somehow become a secondary carbocation, it would become more stable. The n-propyl carbocation does indeed turn into a secondary carbocation through the rearrangement of an alpha-hydrogen. This process is called a 1,2-hydride shift since the hydrogen rearranges as a hydride and it occurs from the alpha-carbon (locant 2) to the cationic carbon (locant 1).
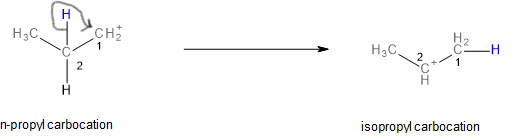
Image: Rearrangement into a more stable, secondary carbocation
The product is a more stable, secondary, isopropyl carbocation.
The isopropyl carbocation is then attacked by a \[\pi \]electron pair of benzene followed by the liberation of hydrogen bromide (\[HBr\]) to form isopropyl benzene which is commonly known as cumene.
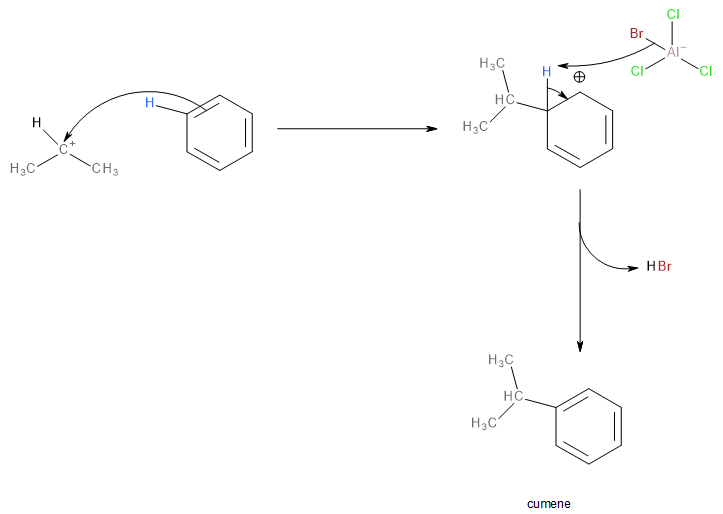
Image: Nucleophilic attack of a benzene ring on the carbocation.
Thus, the correct option is D.
Note: The rearrangement of the primary n-propyl carbocation into a more stable, secondary, isopropyl carbocation via 1,2-hydride shift is a very crucial step in this reaction. This is where quite a few students may go wrong. If they are unaware of this rearrangement step, they might mark option A as the answer to this question. They would be wrong.
Complete Step by Step Solution:
Friedel-Crafts reactions are a set of reactions which involve the attaching of a substituent to an aromatic ring. Friedel-Crafts reactions are of two types, alkylation reactions, in which an alkyl group (\[ - R\]) gets attached to a ring, and acylation reactions involving the attaching of an acyl group (\[ - RC = O\]) to a ring. In this reaction, the catalyst employed is aluminium chloride (\[AlC{l_3}\]) which is a strong Lewis acid. The alkylating agents have traditionally been alkyl halides.
Let’s have a look at how benzene and n-propyl bromide undergo Friedel-Craft’s reaction in presence of aluminium chloride.
In the first step, the n-propyl bromide loses its bromine atom with the help of aluminium chloride as shown. Since aluminium chloride is a strong Lewis acid, it accepts electrons from the bromine atom of n-propyl bromide, and it leads to the formation of an n-propyl carbocation.

Image: Formation of n-propyl carbocation
The n-propyl carbocation formed is primary. We know that primary carbocations are the least stable (order of stability of carbocations: \[1^\circ < 2^\circ < 3^\circ \]). Since secondary carbocations are more stable, if the n-propyl carbocation can somehow become a secondary carbocation, it would become more stable. The n-propyl carbocation does indeed turn into a secondary carbocation through the rearrangement of an alpha-hydrogen. This process is called a 1,2-hydride shift since the hydrogen rearranges as a hydride and it occurs from the alpha-carbon (locant 2) to the cationic carbon (locant 1).

Image: Rearrangement into a more stable, secondary carbocation
The product is a more stable, secondary, isopropyl carbocation.
The isopropyl carbocation is then attacked by a \[\pi \]electron pair of benzene followed by the liberation of hydrogen bromide (\[HBr\]) to form isopropyl benzene which is commonly known as cumene.

Image: Nucleophilic attack of a benzene ring on the carbocation.
Thus, the correct option is D.
Note: The rearrangement of the primary n-propyl carbocation into a more stable, secondary, isopropyl carbocation via 1,2-hydride shift is a very crucial step in this reaction. This is where quite a few students may go wrong. If they are unaware of this rearrangement step, they might mark option A as the answer to this question. They would be wrong.
Recently Updated Pages
JEE Main 2026 Session 2 Registration Open, Exam Dates, Syllabus & Eligibility

JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

Trending doubts
JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding Average and RMS Value in Electrical Circuits

Understanding Collisions: Types and Examples for Students

Ideal and Non-Ideal Solutions Explained for Class 12 Chemistry

Understanding Atomic Structure for Beginners

AssertionIn electrolytic refining of metal impure metal class 12 chemistry JEE_Main

Other Pages
JEE Advanced Weightage 2025 Chapter-Wise for Physics, Maths and Chemistry

NCERT Solutions For Class 12 Chemistry Chapter 2 Solutions Hindi Medium (2025-26)

CBSE Class 12 Chemistry Set 1 56/2/1 2025: Question Paper, Answers & Analysis

CBSE Class 12 Chemistry Question Paper Set 3 2025 with Answers

Inductive Effect and Its Role in Acidic Strength

Degree of Dissociation: Meaning, Formula, Calculation & Uses




