
Give the names of the following compounds.
(i) \[HClO\]
(ii) \[HCl{O_2}\]
(iii) \[HCl{O_3}\]
(iv) \[HCl{O_4}\]
Answer
233.1k+ views
Hint: Chemical name is used to represent any chemical compound. For different chemical compounds, different unique chemical names are applied.
Complete step-by-step answer:
Acids are chemical compounds with a pH of less than 7. Acids generally dissociate when added to water to give hydrogen ions.
(i) \[HClO\]
The structure is shown below.
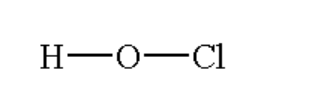
Image: \[HClO\]
Here, one oxygen atom is bonded to one hydrogen atom and one chlorine atom.
The chemical name of \[HClO\]is hypochlorous acid. It is a weak acid.
(ii) \[HCl{O_2}\]
The structure is shown below.
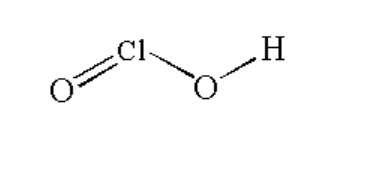
Image: \[HCl{O_2}\]
Here, the chlorine atom is bonded with an oxygen atom by a double bond and one oxygen atom by a single bond and one oxygen atom is bonded with hydrogen by a single bond.
The chemical name of \[HCl{O_2}\]chlorous acid. It is a oxoacid of chlorine.
(iii) \[HCl{O_3}\]
The structure is shown below.
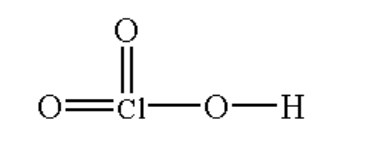
Image: \[HCl{O_3}\]
Here the chlorine atom is bonded with two oxygen atoms by a double bond and one oxygen atom by a single bond and the oxygen atom is bonded with hydrogen by a single bond.
The chemical name of \[HCl{O_3}\] is chloric acid.It is a colourless liquid.
(iv) \[HCl{O_4}\]
The structure is shown below.
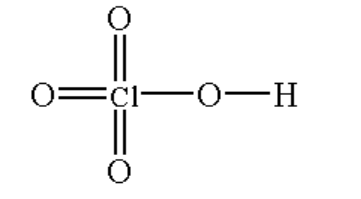
Image: \[HCl{O_4}\]
Here, the chlorine atom is bonded with three oxygen atoms by a double bond and one oxygen atom by a single bond and the oxygen atom is bonded with hydrogen by a single bond.
The chemical name of \[HCl{O_4}\]is perchloric acid. It is a colourless, odourless solution.
Note: The given chemical compounds, hypochlorous acid, chlorous acid, chloric acid, and perchloric acid come under chlorine acid. Hydrochloric acid also come under chlorine acid.
Complete step-by-step answer:
Acids are chemical compounds with a pH of less than 7. Acids generally dissociate when added to water to give hydrogen ions.
(i) \[HClO\]
The structure is shown below.
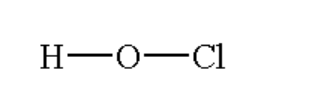
Image: \[HClO\]
Here, one oxygen atom is bonded to one hydrogen atom and one chlorine atom.
The chemical name of \[HClO\]is hypochlorous acid. It is a weak acid.
(ii) \[HCl{O_2}\]
The structure is shown below.

Image: \[HCl{O_2}\]
Here, the chlorine atom is bonded with an oxygen atom by a double bond and one oxygen atom by a single bond and one oxygen atom is bonded with hydrogen by a single bond.
The chemical name of \[HCl{O_2}\]chlorous acid. It is a oxoacid of chlorine.
(iii) \[HCl{O_3}\]
The structure is shown below.
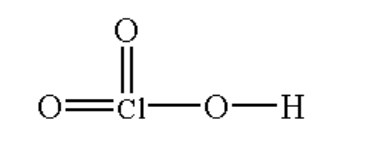
Image: \[HCl{O_3}\]
Here the chlorine atom is bonded with two oxygen atoms by a double bond and one oxygen atom by a single bond and the oxygen atom is bonded with hydrogen by a single bond.
The chemical name of \[HCl{O_3}\] is chloric acid.It is a colourless liquid.
(iv) \[HCl{O_4}\]
The structure is shown below.

Image: \[HCl{O_4}\]
Here, the chlorine atom is bonded with three oxygen atoms by a double bond and one oxygen atom by a single bond and the oxygen atom is bonded with hydrogen by a single bond.
The chemical name of \[HCl{O_4}\]is perchloric acid. It is a colourless, odourless solution.
Note: The given chemical compounds, hypochlorous acid, chlorous acid, chloric acid, and perchloric acid come under chlorine acid. Hydrochloric acid also come under chlorine acid.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




