
Formalin is the commercial name of
A. Formic acid
B. Fluoroform
C. \[40\% \] aqueous solution of methanol
D. Paraformaldehyde
Answer
232.8k+ views
Hint: Formaldehyde is the simplest aldehyde made of hydrogen, carbon and oxygen. It is one of a large family of chemicals known as volatile organic compounds which evaporates and becomes gaseous at room temperature.
Complete step by step answer:
Formaldehyde also known as methanal is a reactive molecule and first in the series of aliphatic aldehydes. Its chemical formula is $C{H_2}O$. Its structure is a shown:
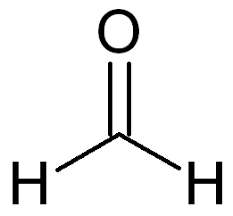
However, it is formed primarily by methanol vapor-phase oxidation, and is commonly sold as formalin, an aqueous solution of $40\% $ methanal. So, a saturated water solution of about $40\% $ formaldehyde by volume or $37\% $ by mass, is called formalin. Since, $100\% $formalin contains up to $15\% $ of methanol as a stabilizer, it has a significant impact on cell fixation.
Moreover, formaldehyde reacts with a base like sodium hydroxide and forms sodium formate and methanol. The reaction is as shown:
$2HCHO + NaOH \to HCOONa + C{H_3}OH$
It also reacts with ammonia and forms formamidine and water. The chemical equation is as shown:
$6HCHO + 4N{H_3} \to (C{H_2})6{N_4} + 6{H_2}O$
Hence, option C is correct.
Note:
Formalin is commonly used as an industrial disinfectant, and as a preservative in funeral homes and medical labs. It can also be used as a preservative in some foods and in products such as antiseptics, medicines and cosmetics. It is also used as an adulterant in milk to increase its shelf life for long distance transportation without refrigeration.
Complete step by step answer:
Formaldehyde also known as methanal is a reactive molecule and first in the series of aliphatic aldehydes. Its chemical formula is $C{H_2}O$. Its structure is a shown:

However, it is formed primarily by methanol vapor-phase oxidation, and is commonly sold as formalin, an aqueous solution of $40\% $ methanal. So, a saturated water solution of about $40\% $ formaldehyde by volume or $37\% $ by mass, is called formalin. Since, $100\% $formalin contains up to $15\% $ of methanol as a stabilizer, it has a significant impact on cell fixation.
Moreover, formaldehyde reacts with a base like sodium hydroxide and forms sodium formate and methanol. The reaction is as shown:
$2HCHO + NaOH \to HCOONa + C{H_3}OH$
It also reacts with ammonia and forms formamidine and water. The chemical equation is as shown:
$6HCHO + 4N{H_3} \to (C{H_2})6{N_4} + 6{H_2}O$
Hence, option C is correct.
Note:
Formalin is commonly used as an industrial disinfectant, and as a preservative in funeral homes and medical labs. It can also be used as a preservative in some foods and in products such as antiseptics, medicines and cosmetics. It is also used as an adulterant in milk to increase its shelf life for long distance transportation without refrigeration.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




