
For an ideal gas, in an isothermal process
A. Heat content remains constant
B. Heat content and temperature remains constant
C. Temperature remains constant
D. None of the above
Answer
240.6k+ views
Hint: In this problem, to determine which option is correct for an isothermal thermodynamic process, we must know the basic fundamentals and applications of an isothermal process following the condition $\Delta T = 0$ hence, use this condition to state the answer for the given situation with proper explanation.
Complete step by step solution:
The term "isothermal process" refers to a substance, an item, or a system changing at a specific constant temperature. Isothermal Process in thermodynamics is a process during which the temperature $T$ of a system remains constant whereas other variables like pressure $P$ and volume $V$ may change that’s why it is also referred to as a constant-temperature process.
In the Isothermal process, $\text{Change in Temperature} = \Delta T = 0$ or, $T{\text{ }} = \text{constant}$. Graphically, an isothermal process can be represented as: -
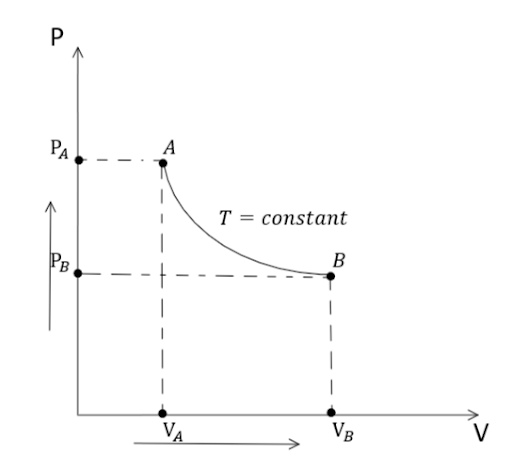
A thermodynamic process is known as an isothermal process when the system temperature is constant because heat is transferred into or out of the system so slowly, that thermal equilibrium is preserved.
If a system comes into contact with a thermal reservoir from the outside, it gradually modifies its own temperature through heat exchange to preserve thermal equilibrium which means heat content is not constant as a result of which options (A) and (B) become incorrect. Thus, in an isothermal process, the temperature remains constant for an ideal gas.
Hence, the correct option is C.
Note: In this case, we know that temperature varies with the given conditions of the system and surroundings. But to keep the system’s temperature constant, either the heat is drawn into the system from the surroundings or it is taken from the system and discharged outside to the surroundings. Additionally, the heat pump is one of the illustrations of an isothermal process.
Complete step by step solution:
The term "isothermal process" refers to a substance, an item, or a system changing at a specific constant temperature. Isothermal Process in thermodynamics is a process during which the temperature $T$ of a system remains constant whereas other variables like pressure $P$ and volume $V$ may change that’s why it is also referred to as a constant-temperature process.
In the Isothermal process, $\text{Change in Temperature} = \Delta T = 0$ or, $T{\text{ }} = \text{constant}$. Graphically, an isothermal process can be represented as: -

A thermodynamic process is known as an isothermal process when the system temperature is constant because heat is transferred into or out of the system so slowly, that thermal equilibrium is preserved.
If a system comes into contact with a thermal reservoir from the outside, it gradually modifies its own temperature through heat exchange to preserve thermal equilibrium which means heat content is not constant as a result of which options (A) and (B) become incorrect. Thus, in an isothermal process, the temperature remains constant for an ideal gas.
Hence, the correct option is C.
Note: In this case, we know that temperature varies with the given conditions of the system and surroundings. But to keep the system’s temperature constant, either the heat is drawn into the system from the surroundings or it is taken from the system and discharged outside to the surroundings. Additionally, the heat pump is one of the illustrations of an isothermal process.
Recently Updated Pages
Dimensions of Charge: Dimensional Formula, Derivation, SI Units & Examples

How to Calculate Moment of Inertia: Step-by-Step Guide & Formulas

Circuit Switching vs Packet Switching: Key Differences Explained

Dimensions of Pressure in Physics: Formula, Derivation & SI Unit

JEE Extractive Metallurgy Important Concepts and Tips for Exam Preparation

JEE General Topics in Chemistry Important Concepts and Tips

Trending doubts
JEE Main 2026: Session 1 Results Out and Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

Ideal and Non-Ideal Solutions Explained for Class 12 Chemistry

JEE Main Participating Colleges 2026 - A Complete List of Top Colleges

Clemmensen and Wolff Kishner Reductions Explained for JEE & NEET

Degree of Dissociation: Meaning, Formula, Calculation & Uses

Understanding the Angle of Deviation in a Prism

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

JEE Advanced 2026 - Exam Date (Released), Syllabus, Registration, Eligibility, Preparation, and More

CBSE Notes Class 11 Physics Chapter 4 - Laws of Motion - 2025-26

CBSE Notes Class 11 Physics Chapter 14 - Waves - 2025-26

CBSE Notes Class 11 Physics Chapter 9 - Mechanical Properties of Fluids - 2025-26

Inductive Effect and Its Role in Acidic Strength




