
How many of the following compounds has $X-X$ bonds i.e. single covalent bond between two same elements in their molecule?
$\begin{align}
& A.{{H}_{4}}{{P}_{2}}{{O}_{6}} \\
& B.{{H}_{4}}{{P}_{2}}{{O}_{5}} \\
& C.{{H}_{4}}{{P}_{2}}{{O}_{7}} \\
& D.{{N}_{2}}O \\
& E.{{N}_{2}}{{O}_{3}} \\
& F.{{N}_{2}}{{O}_{4}} \\
& G.{{N}_{2}}{{O}_{5}} \\
& H.{{(HP{{O}_{3}})}_{3}} \\
\end{align}$
Answer
232.8k+ views
Hint: Draw the structure of all the molecules given in the options and then count the number of single covalent bonds in there respective structures and then that will be the correct answers
Complete Step by Step Answer:
The hypophosphoric acids of the phosphorus element has many times peroxo linkage in their structures. The method of drawing the structures is to attach all the hydrogen as hydroxo ion i.e OH bonds and rest oxygen is connected as per the covalency of the central atom like phosphorus in the phospohoric acids and nitrogen in the nitrous compounds given . So , first we need to draw the diagrams of all the compounds given above.
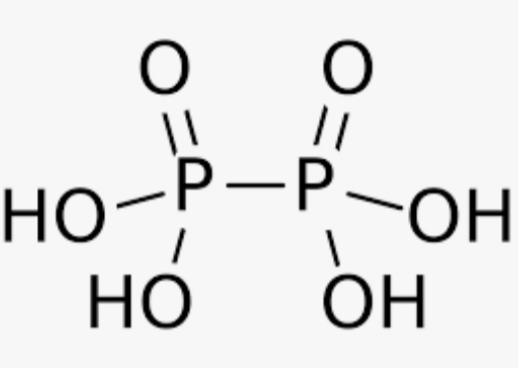
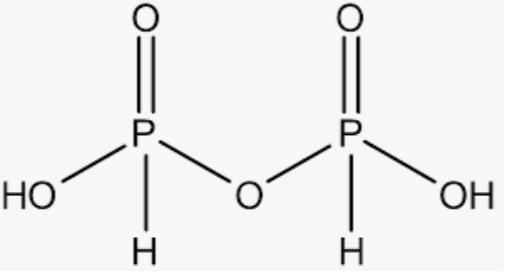
${{(HP{{O}_{3}})}_{3}}$ ${{(HP{{O}_{3}})}_{3}}$
From these structures we can infer that the compounds in which the single covalent bond is present in the same element are 3 compounds .
Thus , the correct option is A,E,F.
Note: Solubility product is a type of equilibrium constant whose value depends on the temperature. It is denoted by ${{K}_{sp}}$. It usually increases with the increase in temperature because of the increased solubility. Remember that solubility and the solubility product are different from each other. Solubility of a substance in a solvent is the highest amount of solute that can be dissolved in a solvent whereas the solubility product is an equilibrium constant that gives the equilibrium between the solid solute and its ions that are dissolved in the solution
Complete Step by Step Answer:
The hypophosphoric acids of the phosphorus element has many times peroxo linkage in their structures. The method of drawing the structures is to attach all the hydrogen as hydroxo ion i.e OH bonds and rest oxygen is connected as per the covalency of the central atom like phosphorus in the phospohoric acids and nitrogen in the nitrous compounds given . So , first we need to draw the diagrams of all the compounds given above.


${{(HP{{O}_{3}})}_{3}}$ ${{(HP{{O}_{3}})}_{3}}$
From these structures we can infer that the compounds in which the single covalent bond is present in the same element are 3 compounds .
Thus , the correct option is A,E,F.
Note: Solubility product is a type of equilibrium constant whose value depends on the temperature. It is denoted by ${{K}_{sp}}$. It usually increases with the increase in temperature because of the increased solubility. Remember that solubility and the solubility product are different from each other. Solubility of a substance in a solvent is the highest amount of solute that can be dissolved in a solvent whereas the solubility product is an equilibrium constant that gives the equilibrium between the solid solute and its ions that are dissolved in the solution
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




